
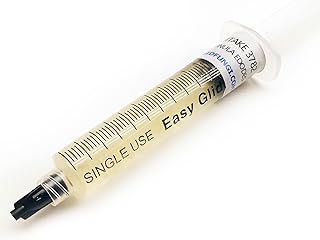
Spore syringes are essential tools in mycology, designed to facilitate the propagation of mushrooms by dispersing spores in a controlled manner. These syringes contain a sterile solution of mushroom spores suspended in water, allowing for precise inoculation of substrates like agar or grain spawn. The process begins by injecting the spore solution into a sterilized growth medium, where the spores germinate and develop into mycelium, the vegetative part of the fungus. This method ensures a higher success rate in cultivation compared to traditional spore dispersal techniques, as it minimizes contamination and provides optimal conditions for spore germination. Understanding how spore syringes work is crucial for both amateur and professional cultivators seeking to grow mushrooms efficiently and reliably.
| Characteristics | Values |
|---|---|
| Purpose | Used to inoculate substrate with mushroom spores for cultivation. |
| Contents | Sterile water solution containing mushroom spores. |
| Material | Typically made of glass or plastic with a Luer lock or slip tip. |
| Sterility | Sterilized to prevent contamination from bacteria or other fungi. |
| Storage | Stored in a cool, dark place to maintain spore viability. |
| Shelf Life | Can last 6 months to 2 years if stored properly. |
| Inoculation Method | Spores are injected into a sterilized substrate (e.g., grain or agar). |
| Contamination Risk | Low if handled in a sterile environment; high if exposed to contaminants. |
| Legal Status | Legal in many regions for microscopy or research; cultivation laws vary. |
| Cost | Typically $10–$30 USD per syringe, depending on species and supplier. |
| Effectiveness | High success rate when used correctly with sterile techniques. |
| Compatibility | Works with various substrates like grain jars, agar plates, or bulk grow kits. |
| Volume | Usually contains 10–20 mL of spore solution. |
| Microscopy Use | Commonly used for studying spore morphology under a microscope. |
| Environmental Impact | Minimal if disposed of properly; avoid releasing spores into the wild. |
Explore related products
$20.63 $22.95
What You'll Learn
- Spore Syringe Composition: Contains spores, water, sometimes nutrients, suspended for easy inoculation into substrate
- Sterilization Process: Ensures syringe and spores are free from contaminants before use
- Injection Technique: Properly inserting spores into substrate using a needle and syringe
- Storage Guidelines: Keep refrigerated, dark, and sealed to maintain spore viability long-term
- Contamination Risks: Common issues like bacteria or mold and how to avoid them

Spore Syringe Composition: Contains spores, water, sometimes nutrients, suspended for easy inoculation into substrate
Spore syringes are a cornerstone of mycology, offering a simple yet effective method for propagating fungi. At their core, these syringes contain a suspension of spores in water, often with added nutrients to enhance viability. This composition is meticulously designed to ensure that spores remain viable and evenly distributed, ready for inoculation into a substrate. The water acts as a medium to keep the spores suspended, preventing them from clumping together and ensuring a consistent distribution when injected. Nutrients, when included, provide an energy source that can extend the spores' shelf life and improve germination rates, though their presence is not always necessary depending on the intended use.
The process of creating a spore syringe begins with spore collection, typically from a mature mushroom cap. These spores are then mixed with sterile water, often distilled or deionized to avoid contamination. The solution is gently agitated to ensure even dispersion, and the syringe is sealed to maintain sterility. For those adding nutrients, common choices include simple sugars like dextrose or more complex mixtures tailored to specific fungal species. The concentration of spores in the solution is critical; a typical dosage ranges from 10 to 50 million spores per milliliter, though this can vary based on the species and intended application. Too few spores may result in slow colonization, while too many can lead to overcrowding and reduced growth.
Inoculation using a spore syringe is straightforward but requires precision. The syringe is inserted into the substrate—often a sterilized grain or agar medium—and the spore solution is slowly injected. The substrate should be at room temperature to avoid shocking the spores. After inoculation, the substrate is sealed and placed in a controlled environment to encourage mycelial growth. For beginners, it’s advisable to start with a small batch to test the viability of the spores and the effectiveness of the inoculation technique. Common mistakes include injecting too quickly, which can cause the spores to settle unevenly, or using a contaminated syringe, which can ruin the entire process.
Comparatively, spore syringes offer advantages over other inoculation methods, such as spore prints or liquid cultures. Spore prints, while traditional, can be less reliable due to uneven spore distribution and susceptibility to contamination. Liquid cultures, on the other hand, often require more advanced techniques and equipment. Spore syringes strike a balance, offering ease of use and reliability, making them ideal for both novice and experienced cultivators. Their portability and shelf life—typically several months when stored properly—further enhance their appeal.
In practice, the composition of a spore syringe is a delicate balance of science and art. The inclusion of nutrients, for instance, can be tailored to specific fungal species, with some benefiting from additional sugars or vitamins. For example, *Psilocybe cubensis* spores often thrive with a simple dextrose solution, while other species may require more complex additives. Proper storage is equally important; syringes should be kept in a cool, dark place, and refrigeration can extend their viability. For those new to mycology, starting with a commercially prepared syringe can provide a reliable foundation before experimenting with custom compositions. Ultimately, understanding the nuances of spore syringe composition empowers cultivators to optimize their techniques and achieve consistent, successful results.
Does Spore Support Controllers? A Guide to Gameplay Options
You may want to see also

Sterilization Process: Ensures syringe and spores are free from contaminants before use
Sterilization is the cornerstone of spore syringe efficacy, ensuring that both the syringe and the spores remain uncontaminated before use. Without this critical step, the introduction of foreign bacteria, fungi, or other microorganisms could compromise the entire cultivation process. The goal is to create an environment where only the desired spores can thrive, free from competition or interference. This process typically involves heat, chemicals, or a combination of both to eliminate any potential contaminants.
One of the most common sterilization methods for spore syringes is autoclaving, which uses high-pressure steam at temperatures around 121°C (250°F) for at least 15–20 minutes. This method is highly effective at killing spores, bacteria, and other microorganisms that might be present on the syringe or in the spore solution. For home cultivators, pressure cookers can serve as a practical alternative to professional autoclaves, provided they reach and maintain the required temperature and pressure. It’s crucial to ensure that all components—syringes, needles, and spore solutions—are properly sealed in sterile containers before autoclaving to prevent recontamination.
Chemical sterilization is another option, often using agents like ethanol or hydrogen peroxide. For instance, wiping down syringes with 70% isopropyl alcohol can reduce surface contaminants, though it’s less reliable than autoclaving for complete sterilization. Hydrogen peroxide, particularly in its vaporized form, is another effective method, often used in commercial settings. However, chemical sterilization is generally more suited for surfaces and tools rather than the spore solution itself, as residual chemicals could harm the spores.
A lesser-known but effective technique is dry heat sterilization, which involves heating components in an oven at temperatures above 160°C (320°F) for several hours. This method is particularly useful for glass syringes or metal needles that can withstand high temperatures without degradation. However, it’s not suitable for spore solutions, as prolonged heat exposure can damage the spores. Each method has its advantages and limitations, and the choice depends on available equipment and the specific needs of the cultivation process.
Proper sterilization isn’t just about eliminating contaminants—it’s about preserving the integrity of the spores. Over-sterilization or improper techniques can render spores inactive, defeating the purpose of the process. For example, exposing spores to temperatures above 150°C (302°F) for extended periods can reduce their viability. Cultivators must strike a balance, ensuring thorough sterilization without compromising spore health. Always follow established protocols and test the viability of spores post-sterilization to confirm success. Mastery of the sterilization process is essential for anyone working with spore syringes, as it directly impacts the outcome of the cultivation endeavor.
Do Spore Mines Charge on the Turn They Drop? Explained
You may want to see also

Injection Technique: Properly inserting spores into substrate using a needle and syringe
The injection technique is a delicate process that requires precision and care to ensure successful colonization of the substrate by the spores. Using a needle and syringe, cultivators can introduce spores directly into the substrate, bypassing the need for traditional methods like spore prints or liquid cultures. This method is particularly useful for inoculating sterile substrates, such as grain or agar, which serve as the foundation for mushroom growth.
Steps for Proper Injection
Begin by preparing your materials: a sterile spore syringe, a flame source (e.g., alcohol lamp or lighter), and your chosen substrate. Ensure the substrate is at room temperature and properly hydrated. Hold the syringe by its middle, avoiding contact with the needle or plunger to maintain sterility. Flame-sterilize the needle by passing it through the flame until it glows red, then allow it to cool for a few seconds. Insert the needle into the substrate at a 45-degree angle, penetrating about 1-2 inches deep, depending on the substrate's density. Slowly depress the plunger to release 1-2 milliliters of spore solution, creating a small pocket of inoculant within the substrate. Withdraw the needle smoothly, and flame-sterilize it again before repeating the process in another location to ensure even distribution.
Cautions and Troubleshooting
Contamination is the primary risk during injection. Always work in a clean environment, and consider using a still air box or laminar flow hood for added protection. If the substrate is too dense, the needle may clog; gently agitate the syringe or use a larger gauge needle if necessary. Over-injection can lead to pooling of the spore solution, which may cause anaerobic conditions and hinder growth. If you notice mold or unusual colors after a few days, discard the substrate, as it likely indicates contamination.
Practical Tips for Success
For best results, inject the substrate within 24 hours of sterilization to minimize the risk of contamination. Label each injection site with a marker or sticker to track progress. Maintain optimal incubation conditions—temperatures between 70-75°F (21-24°C) and high humidity—to encourage rapid colonization. After injection, seal the substrate container with micropore tape to allow gas exchange while preventing contaminants from entering. Patience is key; colonization can take 1-4 weeks, depending on the mushroom species and substrate type.
Comparative Advantage of Injection
Compared to traditional methods, the injection technique offers greater control over spore distribution and reduces the risk of surface contamination. It is particularly advantageous for small-scale cultivators or those working with sensitive species. While it requires more precision, the higher success rate and efficiency make it a valuable skill for any mycologist. Mastery of this technique opens doors to more advanced cultivation methods, such as grain spawn production and agar work.
How to Fix Spore Transfer Issues to Origin: A Comprehensive Guide
You may want to see also
Explore related products

Storage Guidelines: Keep refrigerated, dark, and sealed to maintain spore viability long-term
Proper storage is critical for preserving the viability of spores in syringes, ensuring they remain potent for cultivation when needed. The key factors—refrigeration, darkness, and sealing—work in tandem to create an environment that minimizes degradation. Refrigeration, ideally between 2°C and 8°C (36°F to 46°F), slows metabolic processes and enzymatic activity that could damage spores. Darkness prevents exposure to light, which can trigger photodegradation and reduce viability. Sealing the syringe in an airtight container protects against moisture fluctuations and contaminants, maintaining the sterile integrity of the spores. Together, these measures extend the shelf life of spore syringes from months to years, depending on the species.
Refrigeration is not just a recommendation but a necessity for long-term storage. Spores, though resilient, are not invincible. At room temperature, their viability diminishes more rapidly due to increased metabolic activity and potential microbial contamination. For optimal results, store syringes in the refrigerator’s main compartment, not the door, to avoid temperature fluctuations. Label the container with the date and spore species to track freshness, as viability can vary—some species remain viable for up to 2 years, while others may degrade after 6 months. Avoid freezing, as ice crystals can rupture spore cell walls, rendering them unusable.
Light exposure is a silent killer of spore viability. Even brief periods of light, especially UV rays, can cause DNA damage and reduce germination rates. To mitigate this, store syringes in opaque containers or wrap them in aluminum foil before refrigeration. For added protection, designate a specific drawer or box in the refrigerator solely for spore syringes, minimizing the risk of accidental light exposure during retrieval. This simple step can significantly enhance the longevity of your spores, ensuring they remain viable for future use.
Sealing is the final line of defense against environmental threats. Airtight containers or vacuum-sealed bags prevent moisture ingress, which can lead to mold or bacterial growth. If using a standard container, include a desiccant packet to absorb excess humidity. For syringes with Luer lock caps, ensure they are tightly secured to prevent leakage or contamination. Periodically inspect seals for integrity, replacing containers or caps if compromised. Proper sealing not only preserves spore viability but also maintains sterility, a crucial factor for successful cultivation.
In practice, combining these storage guidelines creates a fail-safe system for spore preservation. For instance, a mycologist storing *Psilocybe cubensis* spores might refrigerate them at 4°C in a light-proof box, sealed with a desiccant, and labeled with the date and species. This approach ensures the spores remain viable for up to 2 years, ready for inoculation when needed. By understanding the science behind refrigeration, darkness, and sealing, cultivators can safeguard their spore syringes, maximizing their investment and minimizing waste.
Does Spore Effectively Work on Grass-Type Pokémon? A Detailed Analysis
You may want to see also

Contamination Risks: Common issues like bacteria or mold and how to avoid them
Spore syringes are delicate tools, and their success hinges on maintaining sterility. Contamination, often from bacteria or mold, can derail your entire process. These microscopic invaders thrive in the nutrient-rich environment spore syringes provide, quickly outcompeting the desired fungi. Understanding the risks and implementing preventive measures is crucial for achieving consistent, healthy results.
Bacteria and mold are opportunistic, exploiting any weakness in your sterilization and handling procedures. A single spore of mold, invisible to the naked eye, can multiply rapidly, consuming the nutrients meant for your desired fungi and producing toxins that inhibit their growth. Similarly, bacteria can form biofilms, protective matrices that shield them from sterilization attempts and further compromise your culture.
The battle against contamination begins with meticulous sterilization. Autoclaving, a process using high-pressure steam, is the gold standard for sterilizing spore syringes and related equipment. Aim for a minimum of 15 minutes at 121°C (250°F) to ensure complete eradication of microorganisms. For heat-sensitive materials, consider chemical sterilization with a 70% isopropyl alcohol solution, but be aware that this method may not be as effective against all types of contaminants.
Beyond sterilization, aseptic technique is paramount. Work in a clean, clutter-free environment, preferably with a laminar flow hood to create a sterile air current. Wear sterile gloves and a lab coat, and avoid touching any surfaces that haven't been disinfected. When handling the spore syringe, minimize exposure to the environment by working quickly and efficiently.
Even with the best precautions, contamination can occur. Regularly inspect your cultures for signs of trouble: discoloration, unusual odors, or the presence of fuzzy growths are all red flags. If contamination is suspected, isolate the affected culture immediately to prevent spread. Unfortunately, contaminated cultures must be discarded to avoid further issues. Remember, vigilance and strict adherence to sterile practices are your best defenses against the ever-present threat of contamination.
Spice Up Your Spore Game: Tips to Find and Harvest Spice
You may want to see also
Frequently asked questions
A spore syringe is a sterile syringe filled with a suspension of mushroom spores in distilled water. It works by delivering these spores to a substrate (like agar or grain) where they can germinate and grow into mycelium, the vegetative part of the fungus, which eventually produces mushrooms.
To use a spore syringe, first sterilize your substrate (e.g., agar or grain jars). Then, shake the syringe to evenly distribute the spores, insert the needle through the injection port or self-healing injection site, and slowly inject the spore solution. Seal the port and incubate the substrate in a warm, dark place to allow mycelium growth.
Spore syringes are legal in many places because they contain only spores, which are not considered a controlled substance. However, cultivating mushrooms from spores may be regulated depending on your location. Always sterilize equipment, work in a clean environment, and follow proper safety protocols to avoid contamination.






![Magic Mushroom Cultivation For Beginners:::: The Ultimate Guide to Successfully "Grow, Harvest, and Use" Mushroom — at Home [DIY Organic Mushroom Farming For Food and Medicine.]](https://m.media-amazon.com/images/I/71G0c0GAY8L._AC_UL320_.jpg)























