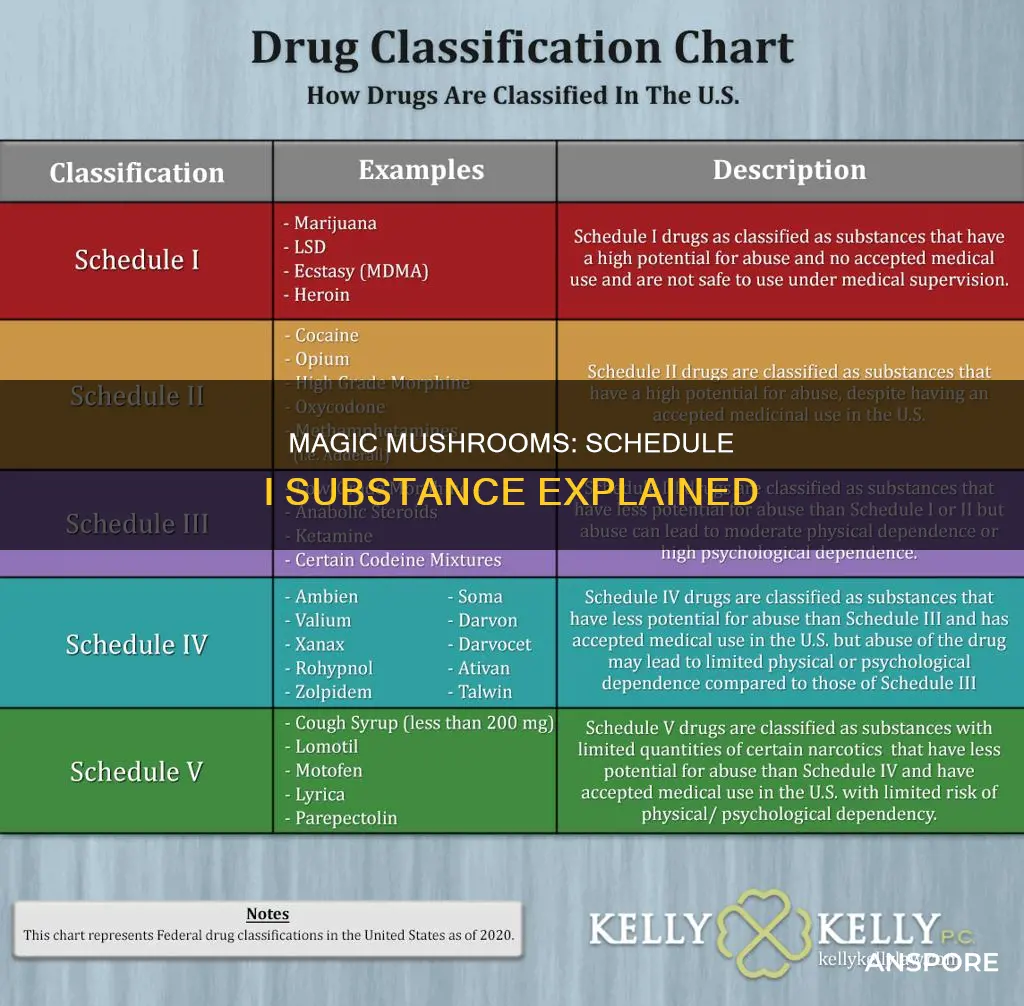
Psilocybin, commonly known as magic mushrooms, is a Schedule I drug under the Controlled Substances Act (CSA) in the United States. Schedule I drugs are defined as drugs with a high potential for abuse and no recognised medical use. Psilocybin mushrooms have been used medicinally and religiously in many cultures throughout history, and some researchers argue that they are less harmful than other Schedule I drugs. Despite this, the possession and use of psilocybin mushrooms are prohibited in most jurisdictions, with varying levels of enforcement and punishment. However, some places, such as the District of Columbia, the Canadian province of Alberta, and the US state of Colorado, have moved to decriminalise or regulate the use of psilocybin mushrooms for medicinal or therapeutic purposes.
| Characteristics | Values |
|---|---|
| Classification | Schedule I drug |
| Other Names | "Magic Mushrooms", Psilocybin, Psilocin |
| Controlled Substances Act (CSA) | Regulates the manufacture, distribution, dispensing, importation, or exportation of any controlled substance |
| Federal Classification | High potential for abuse, no currently accepted medical use in treatment in the U.S., lack of accepted safety for use under medical supervision |
| Legal Status | Varies worldwide, prohibited under most national drug laws, some jurisdictions ban the sale and possession of spores |
| Reclassification Potential | Researchers suggest reclassification to Schedule IV if Phase III clinical trials are successful |
| Therapeutic Benefits | Significant recognition for potential healing benefits in therapeutic settings |
| Risks | Not without risks of harm, but relatively less harmful than other drugs and not prone to compulsive abuse |
Explore related products
What You'll Learn

Psilocybin mushrooms are Schedule I drugs
Psilocybin mushrooms, commonly known as "magic mushrooms", are currently classified as Schedule I drugs under the Controlled Substances Act (CSA) in the United States. Schedule I drugs are defined as substances with a high potential for abuse and no currently accepted medical use in treatment. Psilocybin falls under this category due to its hallucinogenic effects and lack of approved medical applications in the U.S.
According to federal law, Schedule I substances are not approved for medical use and are highly regulated. Physicians and licensed healthcare providers are prohibited from accessing or prescribing these substances. Psilocybin mushrooms are specifically listed as Schedule I drugs under the United Nations 1971 Convention on Psychotropic Substances, which requires member countries to prohibit or strictly control the use of the drug.
While psilocybin mushrooms are classified as Schedule I drugs, there is ongoing research and debate regarding their potential therapeutic benefits. Some organizations, such as the Johns Hopkins Center for Psychedelic & Consciousness Research, have been studying the effectiveness of psilocybin in treating conditions like depression, anxiety, and post-traumatic stress disorder (PTSD). Researchers have suggested that if psilocybin clears phase III clinical trials, it should be reclassified as a Schedule IV drug, similar to prescription sleep aids but with tighter control.
The legal status of psilocybin mushrooms varies across different jurisdictions. In the United States, the possession and use of psilocybin are prohibited under federal law, and most state courts consider the mushroom itself an illegal substance. However, there have been recent moves towards decriminalization and regulated use in certain states, such as Colorado, and in the District of Columbia. Similarly, other countries like Canada and Australia have started to allow the use of psilocybin for medicinal purposes or in prescription medications for treating conditions like PTSD and treatment-resistant depression.
Psychedelic Pink Buffalo Mushrooms: A Trippy Treat?
You may want to see also

They are considered to have a high potential for abuse
Psilocybin, commonly known as "magic mushrooms", is currently classified as a Schedule I drug under the Controlled Substances Act (CSA) in the United States. Schedule I drugs are those that have a high potential for abuse, no currently accepted medical use in treatment, and lack accepted safety for use under medical supervision. Psilocybin mushrooms are illegal to possess and use in most countries, and their legal status varies worldwide.
In the United States, the federal classification of psilocybin as a Schedule I drug means that it is illegal for physicians and licensed healthcare providers to access or prescribe it. However, psilocybin has gained significant recognition for its potential therapeutic benefits when used in a controlled setting. While it is not prone to compulsive abuse, researchers recommend that its use should be tightly controlled and administered in a healthcare setting by trained personnel to minimize potential harm and abuse.
The United Nations Convention on Psychotropic Substances, adopted in 1971, requires its members to prohibit psilocybin. However, the mushrooms containing the drug were not specifically included in the convention due to pressure from the Mexican government. While the possession and use of psilocybin are prohibited under almost all circumstances globally, there have been recent moves towards decriminalization and regulated use in some jurisdictions. For example, in 2020, the District of Columbia passed the Entheogenic Plant and Fungus Policy Act, allowing the possession and non-profit distribution of psilocybin mushrooms.
In summary, psilocybin mushrooms are considered to have a high potential for abuse, and their possession and use are tightly regulated or prohibited in most countries. However, there is growing recognition of their potential therapeutic benefits, and some jurisdictions are exploring decriminalization and regulated use for medicinal purposes. Researchers recommend that any use of psilocybin mushrooms should be tightly controlled to minimize potential harm and abuse.
Magic Mushrooms and THC: A Psychedelic Mix?
You may want to see also

They are not approved for medical treatment in the US
Psilocybin mushrooms, also known as magic mushrooms, are considered Schedule I drugs under the United Nations 1971 Convention on Psychotropic Substances. Schedule I drugs are defined as drugs with a high potential for abuse and no recognized medical uses. According to federal law in the US, no prescriptions may be written for Schedule I substances, and they are not approved for medical treatment.
While there is a growing body of research and clinical trials exploring the therapeutic potential of psilocybin mushrooms for treating mental health disorders such as anxiety, depression, and obsessive-compulsive disorder, there are currently no approved therapeutic products containing psilocybin in the US or elsewhere. The safety, efficacy, and quality of psilocybin products have not been assessed by the relevant health authorities and they have not undergone the rigorous scientific review process required for authorization.
In the US, the possession and sale of psilocybin mushrooms violate federal law and can result in severe legal penalties. Most US state courts have considered the mushroom a "container" of illicit drugs, making it illegal. However, there has been ambiguity and selective enforcement in some jurisdictions. For example, spores of psilocybin mushrooms, which do not contain the psychoactive compounds, may be legal to possess in certain areas.
While some US states have legalized marijuana for personal, recreational, or medical use, psilocybin mushrooms remain a Schedule I substance at the federal level. Local legalization and decriminalization efforts, such as the ballot initiative passed in Oregon in 2020, do not change the Controlled Substance Act, and the immigration consequences for noncitizens remain unchanged. Therefore, it is important for individuals, especially noncitizens, to be aware of the legal risks associated with the use or possession of psilocybin mushrooms in the US.
Mushrooms: Nutritional Powerhouses or Not?
You may want to see also
Explore related products

They are illegal in most countries
Psilocybin, commonly known as "magic mushrooms", is currently classified as a Schedule I drug under the Controlled Substances Act (CSA) in the United States. Schedule I drugs are defined as substances with a high potential for abuse and no recognised medical uses or therapeutic benefits. Psilocybin mushrooms are also listed as Schedule I drugs per the United Nations 1971 Convention on Psychotropic Substances.
The possession and use of psilocybin are prohibited under almost all circumstances in most countries, often carrying severe legal penalties. Many countries have some level of regulation or prohibition of psilocybin mushrooms, including the US Psychotropic Substances Act, the UK Misuse of Drugs Act 1971, the Canadian Controlled Drugs and Substances Act, and the Japanese Narcotics and Psychotropics Control Law. In some jurisdictions, psilocybin mushroom spores are legal to possess and sell because they do not contain psilocybin or psilocin, the regulated compounds. However, cultivation is often considered drug manufacture and is severely penalised.
While psilocybin remains a Schedule I drug under federal law in the US, some states have taken steps toward decriminalisation and medical use. In 2020, the District of Columbia passed the Entheogenic Plant and Fungus Policy Act, allowing the possession and non-profit distribution of psilocybin mushrooms. In 2022, Colorado became the second US state to decriminalise psilocybin mushrooms, and Australia approved their use in prescription medications for PTSD and treatment-resistant depression.
Despite the illegal status of psilocybin mushrooms in most countries, there has been growing recognition of their potential therapeutic benefits. Researchers at Johns Hopkins University have suggested that psilocybin could be reclassified as a Schedule IV drug if it clears phase III clinical trials, indicating its potential for medical use with tighter control. However, the drug remains unapproved by the Food and Drug Administration (FDA), and the DEA continues to classify it as a substance with no currently accepted medical use and a high potential for abuse.
Microdosing Mushrooms: Tolerance and Its Impact
You may want to see also

Researchers suggest re-categorising them to Schedule IV
Psilocybin, the chemical compound found in hallucinogenic mushrooms, is currently classified as a Schedule I drug by the United States Drug Enforcement Agency (DEA). This classification is given to substances that have a high potential for abuse, no currently accepted medical use in the U.S., and a lack of accepted safety for use under medical supervision.
However, researchers from Johns Hopkins University School of Medicine have suggested that psilocybin should be reclassified as a Schedule IV drug. This recommendation is based on several factors. Firstly, studies in both animals and humans have shown that psilocybin has a low potential for abuse. In animal studies, rats did not repeatedly self-administer psilocybin in the same way they did for other drugs such as cocaine, alcohol, or heroin. Similarly, in human studies, individuals who have used psilocybin typically report using it a small number of times across their lifetime.
Secondly, psilocybin has demonstrated potential therapeutic benefits in preliminary research studies. It has been suggested that psilocybin may be effective for smoking cessation and for treating disorders such as cancer-specific depression and anxiety. Additionally, psilocybin has been used in traditional ceremonies and religious rituals for thousands of years, indicating a long history of human use.
Finally, psilocybin is relatively less harmful than other drugs. It has the lowest potential for lethal overdose as there is no known overdose level. However, it is important to note that psilocybin is not without risks. Extreme fear, anxiety, panic, or paranoia can occur during a "bad trip". Additionally, there is a risk of misidentifying mushrooms and consuming toxic mushrooms instead of those containing psilocybin.
Despite the potential benefits and low abuse potential, researchers recommend that psilocybin should remain under the supervision of healthcare professionals and be administered in a controlled healthcare setting. They suggest that the reclassification of psilocybin as a Schedule IV drug could facilitate its path to clinical use and minimize logistical hurdles, but tight control and monitoring are still necessary to ensure safe usage.
Mushrooms' Intricate Communication Networks: Unveiling Secrets
You may want to see also
Frequently asked questions
Magic mushrooms, also known as psilocybin mushrooms, are fungi that contain the psychoactive compound psilocybin. They have been used for centuries in various cultures for medicinal and religious purposes.
Psilocybin mushrooms are currently classified as a Schedule I drug by the United Nations 1971 Convention on Psychotropic Substances and the US Drug Enforcement Agency (DEA). Schedule I drugs are considered to have a high potential for abuse and no accepted medical use.
Yes, there have been reclassification recommendations to move psilocybin mushrooms from a Schedule I to a Schedule IV drug. Researchers from Johns Hopkins University suggest that if psilocybin clears phase III clinical trials, it should be reclassified as a Schedule IV drug, similar to prescription sleep aids, but with tighter control.











































