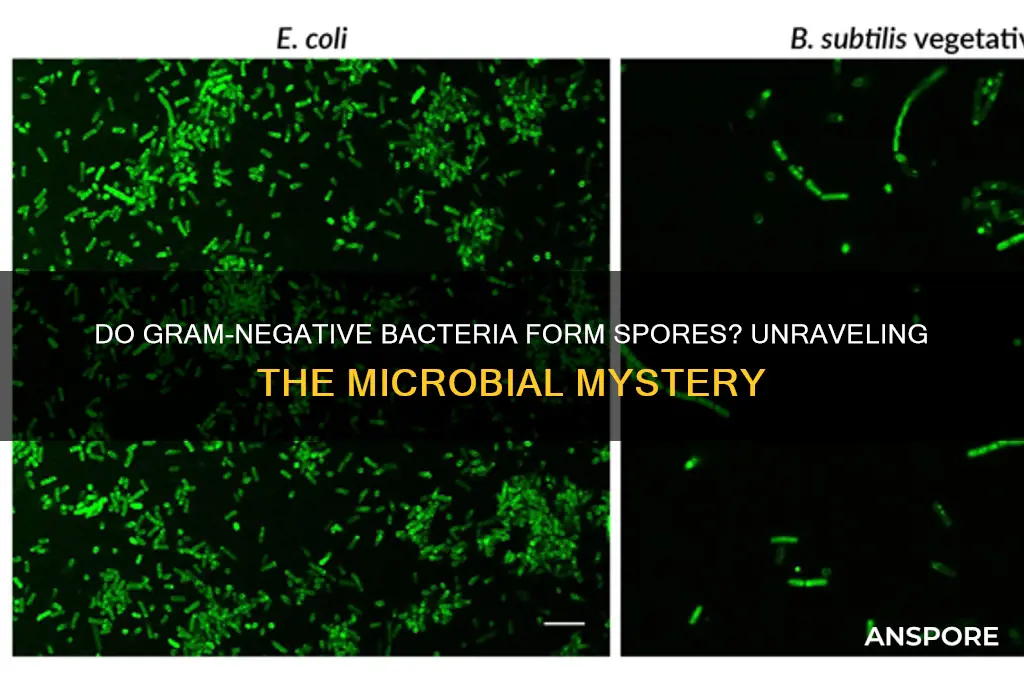
Gram-negative bacteria are a diverse group of microorganisms characterized by their outer membrane containing lipopolysaccharides, which distinguishes them from gram-positive bacteria. While spore formation is a well-known survival mechanism in certain gram-positive bacteria, such as *Bacillus* and *Clostridium*, it is generally rare among gram-negative species. However, there are exceptions, as some gram-negative bacteria, like *Xanthomonas* and *Achromobacter*, have been reported to produce spore-like structures under specific environmental conditions. Understanding whether gram-negative bacteria are spore-forming is crucial for assessing their resilience, pathogenicity, and response to antimicrobial treatments, as spores are highly resistant to harsh conditions and disinfection methods.
| Characteristics | Values |
|---|---|
| Spore Formation | Most Gram-negative bacteria do not form spores. |
| Exceptions | Rare exceptions exist, such as Adenosynbacter and Oceanibacillus. |
| Endospore Formation | Endospores are primarily formed by Gram-positive bacteria. |
| Cell Wall Structure | Gram-negative bacteria have a thin peptidoglycan layer and outer membrane. |
| Survival Mechanisms | Rely on biofilm formation, antibiotic resistance, and other strategies instead of spores. |
| Examples of Non-Spore Formers | Escherichia coli, Pseudomonas aeruginosa, Salmonella spp. |
| Ecological Role | Thrive in diverse environments without spore formation. |
| Medical Significance | Many Gram-negative pathogens are non-spore formers but pose significant health risks. |
Explore related products
What You'll Learn
- Spore Formation Mechanisms: Gram-negative bacteria lack typical spore-forming abilities due to cell wall structure
- Exceptions in Gram-Negatives: Rare species like *Chromobacterium violaceum* exhibit spore-like structures under stress
- Endospore vs. Exospore: Gram-negative bacteria do not produce endospores, unlike some Gram-positives
- Survival Strategies: Gram-negatives rely on biofilms, cysts, or persistence instead of spores for survival
- Laboratory Identification: Absence of spores in Gram-negative bacteria is a key diagnostic feature

Spore Formation Mechanisms: Gram-negative bacteria lack typical spore-forming abilities due to cell wall structure
Gram-negative bacteria are structurally distinct from their Gram-positive counterparts, primarily due to their complex cell wall architecture. This difference is pivotal in understanding why Gram-negative bacteria do not form spores. The Gram-negative cell wall consists of a thin peptidoglycan layer sandwiched between an inner cytoplasmic membrane and an outer membrane. This outer membrane, composed of lipopolysaccharides, acts as a robust barrier but also complicates the spore formation process. Spores require a thick, protective peptidoglycan layer to withstand harsh conditions, a feature Gram-negative bacteria lack. Without this structural foundation, the intricate machinery needed for spore formation cannot be effectively deployed.
Consider the spore-forming process in Gram-positive bacteria like *Bacillus subtilis*. Here, a thick peptidoglycan layer provides the necessary framework for the assembly of spore coats and the encapsulation of DNA. In contrast, Gram-negative bacteria would need to reorganize their entire cell wall structure to accommodate spore formation. This would involve not only thickening the peptidoglycan layer but also integrating additional protective layers, a process that would require significant evolutionary adaptation. Given the energy and resource demands of such changes, it’s unsurprising that Gram-negative bacteria have not evolved this ability.
From a practical standpoint, the inability of Gram-negative bacteria to form spores has implications for sterilization and disinfection protocols. While Gram-positive spores, such as those of *Clostridium botulinum*, can survive extreme conditions like high heat and desiccation, Gram-negative bacteria are generally more susceptible to standard sterilization methods. For instance, autoclaving at 121°C for 15 minutes effectively kills most Gram-negative bacteria, whereas spore-forming Gram-positive bacteria may require longer exposure times or higher temperatures. This distinction is critical in industries like food processing and healthcare, where understanding bacterial resistance mechanisms is essential for safety.
A comparative analysis highlights the evolutionary trade-offs between Gram-negative and Gram-positive bacteria. Gram-negative bacteria have prioritized the development of a robust outer membrane, which confers resistance to many antibiotics and environmental stressors. However, this adaptation has come at the cost of spore-forming ability. Conversely, Gram-positive bacteria have invested in thick peptidoglycan layers, enabling spore formation but leaving them more vulnerable to certain antibiotics. This divergence underscores the principle that evolutionary adaptations are often context-dependent, shaped by the specific challenges organisms face in their environments.
In conclusion, the absence of spore formation in Gram-negative bacteria is a direct consequence of their cell wall structure. While this limits their ability to survive extreme conditions, it also simplifies their eradication in controlled settings. Understanding this mechanism not only sheds light on bacterial biology but also informs practical strategies for managing bacterial contamination. For researchers and practitioners, this knowledge is invaluable for designing effective sterilization protocols and developing targeted antimicrobial therapies.
Unveiling the Unique Appearance of Morel Spores: A Visual Guide
You may want to see also

Exceptions in Gram-Negatives: Rare species like *Chromobacterium violaceum* exhibit spore-like structures under stress
Gram-negative bacteria are typically non-spore forming, a trait that distinguishes them from many Gram-positive species. However, exceptions exist, and *Chromobacterium violaceum* stands out as a rare example. Under conditions of environmental stress, such as nutrient deprivation or desiccation, this bacterium can produce structures resembling spores. These formations, often termed "cysts" or "spore-like bodies," enhance its survival in harsh conditions. While not true endospores like those in *Bacillus* or *Clostridium*, they serve a similar protective function, challenging the generalization that Gram-negatives lack spore-forming capabilities.
Analyzing the mechanism behind *C. violaceum*'s spore-like structures reveals a fascinating adaptation. When stressed, the bacterium undergoes morphological changes, including cell wall thickening and accumulation of storage granules. These modifications increase resistance to heat, UV radiation, and antimicrobial agents. For instance, studies show that such structures can survive in soil for months, a survival strategy akin to spore formation. This adaptability highlights the evolutionary ingenuity of certain Gram-negative species, even if their mechanisms differ from traditional sporulation pathways.
For researchers and clinicians, understanding *C. violaceum*'s unique behavior has practical implications. Misidentification of these spore-like structures could lead to diagnostic errors, as they may be mistaken for true spores under microscopy. Additionally, their resilience complicates disinfection efforts, particularly in healthcare settings. To mitigate risks, laboratories should employ stringent sterilization protocols, such as autoclaving at 121°C for 15–20 minutes, to ensure complete inactivation. Awareness of this exception is crucial for accurate identification and effective management of potential infections.
Comparatively, *C. violaceum*'s spore-like structures differ from true endospores in key ways. Unlike the highly refractory spores of Gram-positives, these structures are more susceptible to certain chemicals and physical agents. For example, while endospores can withstand boiling for hours, *C. violaceum*'s formations are less durable. This distinction underscores the importance of precise terminology and targeted approaches when dealing with such exceptions. Recognizing these nuances ensures appropriate scientific and clinical responses.
In conclusion, *Chromobacterium violaceum* exemplifies the rare ability of certain Gram-negative bacteria to form spore-like structures under stress. This phenomenon, though distinct from true sporulation, showcases the diversity of bacterial survival strategies. For practitioners, acknowledging this exception is vital for accurate diagnosis, effective disinfection, and informed research. By studying such outliers, we gain deeper insights into bacterial resilience and adaptability, challenging conventional assumptions about Gram-negative behavior.
How Long Do Mold Spores Stay Airborne and Pose Risks?
You may want to see also

Endospore vs. Exospore: Gram-negative bacteria do not produce endospores, unlike some Gram-positives
Gram-negative bacteria are known for their distinctive cell wall structure, which includes an outer membrane containing lipopolysaccharides. Despite their resilience in various environments, these bacteria do not produce endospores, a survival mechanism employed by certain Gram-positive species. This distinction is critical in microbiology, as endospores allow bacteria to withstand extreme conditions such as heat, desiccation, and radiation. For instance, *Clostridium botulinum* and *Bacillus anthracis*, both Gram-positive, form endospores that can persist in soil for decades. In contrast, Gram-negative bacteria like *Escherichia coli* and *Pseudomonas aeruginosa* lack this ability, relying instead on other strategies such as biofilm formation to endure harsh conditions.
While Gram-negative bacteria do not form endospores, it is essential to clarify the difference between endospores and exospores. Endospores are highly resistant, dormant structures formed internally within the bacterial cell, primarily by Gram-positive species. Exospores, on the other hand, are less common and typically associated with certain fungi or algae, not bacteria. This distinction highlights the unique survival mechanisms across different microbial groups. For practical purposes, understanding that Gram-negative bacteria do not produce endospores is crucial in fields like food safety and infection control, where spore-forming bacteria pose significant challenges due to their resistance to sterilization methods.
From a clinical perspective, the absence of endospore formation in Gram-negative bacteria simplifies their eradication in medical settings. For example, autoclaving at 121°C for 15 minutes effectively kills vegetative Gram-negative cells, whereas endospore-forming Gram-positive bacteria require more stringent conditions. However, Gram-negative bacteria present other challenges, such as their outer membrane acting as a barrier to many antibiotics. This underscores the importance of targeted antimicrobial strategies based on bacterial characteristics. Researchers and healthcare professionals must remain vigilant, as misidentifying a Gram-negative bacterium as spore-forming could lead to inappropriate treatment protocols.
To illustrate the practical implications, consider the food industry, where spore-forming bacteria like *Bacillus cereus* can survive cooking and cause foodborne illness. In contrast, Gram-negative pathogens such as *Salmonella* and *Shigella* are less likely to survive high-temperature processing due to their inability to form endospores. However, their rapid growth in favorable conditions necessitates proper refrigeration and hygiene practices. For home cooks, this means ensuring meats are cooked to internal temperatures of 75°C (165°F) to eliminate vegetative Gram-negative bacteria effectively. This knowledge empowers individuals to mitigate risks through informed food handling practices.
In summary, the inability of Gram-negative bacteria to produce endospores is a defining trait that differentiates them from certain Gram-positive species. While this limits their survival in extreme conditions, it also simplifies their control in clinical and industrial settings. However, their other virulence factors, such as antibiotic resistance and biofilm formation, require targeted management strategies. By understanding these distinctions, professionals and laypersons alike can better address the challenges posed by Gram-negative bacteria in various contexts.
Shroomish's Spore Move: When and How to Unlock It
You may want to see also
Explore related products
$27.25

Survival Strategies: Gram-negatives rely on biofilms, cysts, or persistence instead of spores for survival
Gram-negative bacteria, unlike their spore-forming counterparts, lack the ability to produce endospores—highly resistant structures that enable survival in extreme conditions. Instead, they employ alternative strategies to endure harsh environments, ensuring their persistence and proliferation. These strategies include the formation of biofilms, the development of cysts, and the adoption of a persistent state, each offering unique advantages for survival.
Biofilms: A Collective Defense Mechanism
One of the most effective survival strategies of gram-negative bacteria is biofilm formation. Biofilms are structured communities of bacteria encased in a self-produced extracellular matrix, often composed of polysaccharides, proteins, and DNA. This matrix acts as a protective barrier, shielding bacteria from antibiotics, host immune responses, and environmental stressors like desiccation and UV radiation. For example, *Pseudomonas aeruginosa*, a notorious gram-negative pathogen, forms biofilms in the lungs of cystic fibrosis patients, making it extremely difficult to eradicate. To combat biofilms, clinicians often prescribe high-dose antibiotic combinations, such as 500 mg of ciprofloxacin twice daily alongside 1 g of ceftazidime every 8 hours, to penetrate the matrix and target embedded bacteria.
Cysts: A Dormant Shield
While less common in gram-negative bacteria compared to protozoa, some species, like *Azotobacter*, form cysts as a survival mechanism. Cysts are dormant, thickened-walled cells that allow bacteria to withstand adverse conditions such as nutrient deprivation or extreme temperatures. Unlike spores, cysts are not as resilient but provide a temporary refuge until conditions improve. For instance, *Azotobacter* cysts can survive in soil for months, reactivating when nutrients become available. This strategy highlights the adaptability of gram-negative bacteria in the absence of spore formation.
Persistence: The Art of Lying Low
Persistence is another critical survival tactic employed by gram-negative bacteria. Persistent cells are a small subpopulation that enter a dormant, non-replicating state in response to stress, such as antibiotic exposure. These cells are not killed by antibiotics targeting active processes like cell wall synthesis or DNA replication. For example, *Escherichia coli* can enter a persistent state when exposed to aminoglycosides, surviving treatment and causing recurrent infections. Clinicians address persistence by extending antibiotic treatment durations, such as administering 2 g of ampicillin every 6 hours for 14 days instead of the standard 7 days, to ensure eradication of these dormant cells.
Practical Takeaways for Managing Gram-Negative Infections
Understanding these survival strategies is crucial for effective infection control. For biofilm-related infections, mechanical disruption, such as wound debridement or catheter removal, should accompany antibiotic therapy. Cyst-forming bacteria may require environmental modifications, like improving soil moisture for agricultural settings. Persistent infections demand prolonged or combination therapies, with close monitoring to prevent relapse. By targeting these specific mechanisms, healthcare providers and researchers can develop more effective strategies to combat gram-negative bacteria, even in the absence of spore formation.
Fungal Spores: Key Players in Reproduction and Survival Strategies
You may want to see also

Laboratory Identification: Absence of spores in Gram-negative bacteria is a key diagnostic feature
In the realm of microbiology, distinguishing between spore-forming and non-spore-forming bacteria is crucial for accurate identification and subsequent treatment. Gram-negative bacteria, a diverse group of microorganisms, are universally recognized for their absence of spore formation, a characteristic that serves as a pivotal diagnostic feature in laboratory settings. This distinction is particularly important when compared to certain Gram-positive bacteria, such as *Clostridium* and *Bacillus*, which are known for their ability to form highly resistant spores.
Analyzing the Diagnostic Process:
Laboratory identification of Gram-negative bacteria involves a series of steps, including Gram staining, biochemical tests, and microscopic examination. The absence of spores is confirmed using techniques like the endospore staining method, where spores appear as bright green bodies under a microscope. For instance, when examining *Escherichia coli* or *Pseudomonas aeruginosa*, technicians consistently observe the lack of spore structures, reinforcing their classification as non-spore-forming organisms. This negative result is as critical as any positive finding, as it narrows down the possible identities of the bacterium and guides further testing.
Practical Instructions for Technicians:
To ensure accurate identification, technicians should follow a structured protocol. Begin with a Gram stain to confirm the bacterium is Gram-negative, then proceed with a spore stain using malachite green and safranin. If no spores are observed, cross-reference this finding with biochemical tests like the oxidase test or API strips. For example, a negative spore stain coupled with a positive oxidase test strongly suggests a Gram-negative bacillus like *Pseudomonas*. Always document findings meticulously, as inconsistencies can lead to misidentification and inappropriate treatment, particularly in clinical settings.
Comparative Insights and Takeaways:
The absence of spores in Gram-negative bacteria not only simplifies their identification but also highlights their ecological and clinical differences from spore-forming counterparts. Unlike *Bacillus anthracis*, which can survive harsh conditions for years due to its spores, Gram-negative bacteria like *Salmonella* rely on rapid replication and environmental adaptability. This distinction is vital in infection control, as spore-forming bacteria require more aggressive disinfection methods, such as autoclaving at 121°C for 15–30 minutes, whereas non-spore-forming bacteria are generally susceptible to standard disinfectants like 70% ethanol.
Persuasive Argument for Clinical Relevance:
Clinicians and researchers must emphasize the absence of spores in Gram-negative bacteria as a cornerstone of diagnostic accuracy. Misidentifying a non-spore-forming bacterium as spore-forming could lead to unnecessary treatments, such as prescribing spore-targeted antibiotics like vancomycin, which are ineffective against Gram-negative organisms. Conversely, understanding this feature allows for targeted therapies, such as using beta-lactams or fluoroquinolones, which are effective against many Gram-negative pathogens. This precision not only improves patient outcomes but also combats the growing issue of antibiotic resistance by avoiding overuse of broad-spectrum agents.
In summary, the absence of spores in Gram-negative bacteria is a definitive diagnostic marker that streamlines laboratory identification and informs clinical decision-making. By mastering this feature and its implications, microbiologists and healthcare professionals can enhance accuracy, efficiency, and patient care.
Are All Bacterial Spores Harmful? Unveiling the Truth Behind Their Nature
You may want to see also
Frequently asked questions
No, most Gram-negative bacteria are not spore-forming. Spore formation is more commonly associated with certain Gram-positive bacteria, such as *Bacillus* and *Clostridium* species.
Yes, a few exceptions exist, such as *Adenosylcobinamide-phosphate synthase* (formerly *Bacillus*) *schlegelii* and some species in the genus *Stenotrophomonas*, but these are rare and not typical of Gram-negative bacteria.
Gram-negative bacteria lack the genetic and structural mechanisms required for spore formation, which is a complex process primarily found in certain Gram-positive bacteria. Their outer membrane and cell wall structure differ significantly from spore-forming species.









![Substances screened for ability to reduce thermal resistance of bacterial spores 1959 [Hardcover]](https://m.media-amazon.com/images/I/51Z99EgARVL._AC_UL320_.jpg)





















