Bacterial spore staining is a critical technique in microbiology used to differentiate between vegetative bacterial cells and highly resistant endospores. One common question that arises is whether the cells observed during spore staining are dead. In reality, the cells within bacterial spores are not dead but rather in a dormant, metabolically inactive state. Endospores are formed by certain bacteria as a survival mechanism in harsh conditions, such as extreme heat, desiccation, or chemical exposure. During spore staining, the vegetative cells are typically killed by heat fixation, while the endospores remain viable due to their robust structure. Thus, the staining process highlights the presence of these resilient spores, which can later germinate and return to an active, vegetative state under favorable conditions. Understanding this distinction is essential for interpreting staining results and appreciating the remarkable survival strategies of bacteria.
| Characteristics | Values |
|---|---|
| Cell Viability | Spores are dormant and metabolically inactive, but not dead. They can revive under favorable conditions. |
| Metabolic Activity | Minimal to no metabolic activity during the spore state. |
| Resistance | Highly resistant to heat, desiccation, radiation, and chemicals due to their thick, protective spore coat. |
| DNA Protection | DNA is protected by a specialized structure called the spore core, which contains dipicolinic acid (DPA) and calcium ions. |
| Germination | Spores can germinate into vegetative cells when exposed to nutrients, moisture, and appropriate environmental conditions. |
| Staining Behavior | Spores stain differently from vegetative cells due to their unique structure; they often appear as refractile bodies in endospore stains. |
| Longevity | Can remain viable for years or even centuries under harsh conditions. |
| Function | Serve as a survival mechanism for bacteria in adverse environments. |
| Detection Method | Detected using specific staining techniques like the Schaeffer-Fulton stain, which differentiates spores from vegetative cells. |
| Revival Process | Requires specific triggers (e.g., nutrients, temperature) to return to the vegetative, actively growing state. |
Explore related products
What You'll Learn
- Spore Formation Process: How bacterial cells transform into spores during adverse environmental conditions
- Spore Stain Techniques: Methods like Schaeffer-Fulton to differentiate spores from vegetative cells
- Spore Dormancy State: Metabolic inactivity and resistance mechanisms in bacterial spores
- Spore Viability Tests: Assessing if spores are alive or dead post-staining
- Spore Germination Factors: Conditions required to reactivate dormant bacterial spores

Spore Formation Process: How bacterial cells transform into spores during adverse environmental conditions
Bacterial spores are not dead cells but rather a dormant, highly resistant form that certain bacteria adopt to survive harsh environmental conditions. This transformation is a remarkable strategy for long-term survival, allowing bacteria to endure extremes of heat, cold, desiccation, and chemicals that would otherwise be lethal. The process of spore formation, or sporulation, is a complex, multi-step cellular program that ensures the bacterium’s genetic material remains intact until conditions improve. Understanding this process is crucial for fields like microbiology, food safety, and medicine, as spores pose challenges in sterilization and infection control.
The spore formation process begins when a bacterial cell senses adverse environmental conditions, such as nutrient depletion. In *Bacillus subtilis*, a well-studied model organism, this triggers the activation of a genetic cascade involving sigma factors, which redirect gene expression toward sporulation. The cell divides asymmetrically, forming a smaller forespore and a larger mother cell. The forespore becomes the future spore, while the mother cell nurtures it, eventually degrading itself to release the mature spore. This altruistic behavior ensures the survival of the bacterium’s genetic lineage.
During sporulation, the forespore undergoes significant structural changes. A thick, multi-layered spore coat forms around the cell membrane, providing resistance to heat, enzymes, and chemicals. Additionally, the bacterium synthesizes dipicolinic acid (DPA), a calcium-chelating molecule that binds to DNA, stabilizing it and protecting it from damage. The water content within the spore decreases dramatically, further enhancing its resistance to desiccation. These adaptations render the spore metabolically inactive but not dead—it remains viable for years or even centuries, awaiting favorable conditions to revert to its vegetative state.
Practical applications of understanding spore formation are abundant. For instance, in food preservation, knowing that spores can survive boiling temperatures (100°C) for extended periods highlights the need for high-pressure processing or chemical treatments to ensure safety. In healthcare, spores of pathogens like *Clostridium difficile* pose risks in hospital settings, necessitating rigorous disinfection protocols. Laboratory techniques, such as spore staining (e.g., the Schaeffer-Fulton method), differentiate spores from vegetative cells using heat and dyes, exploiting the spore’s resistance to demonstrate its unique structure without killing it.
In summary, the spore formation process is a sophisticated survival mechanism, not a state of cell death. By transforming into spores, bacteria ensure their persistence through adverse conditions, posing both challenges and opportunities for human endeavors. Recognizing the dormant yet viable nature of spores is essential for effective control strategies, whether in industrial sterilization or clinical microbiology. This knowledge bridges the gap between theoretical microbiology and practical applications, underscoring the resilience of life at the microbial level.
Are Fern Spores Haploid? Unraveling the Mystery of Fern Reproduction
You may want to see also

Spore Stain Techniques: Methods like Schaeffer-Fulton to differentiate spores from vegetative cells
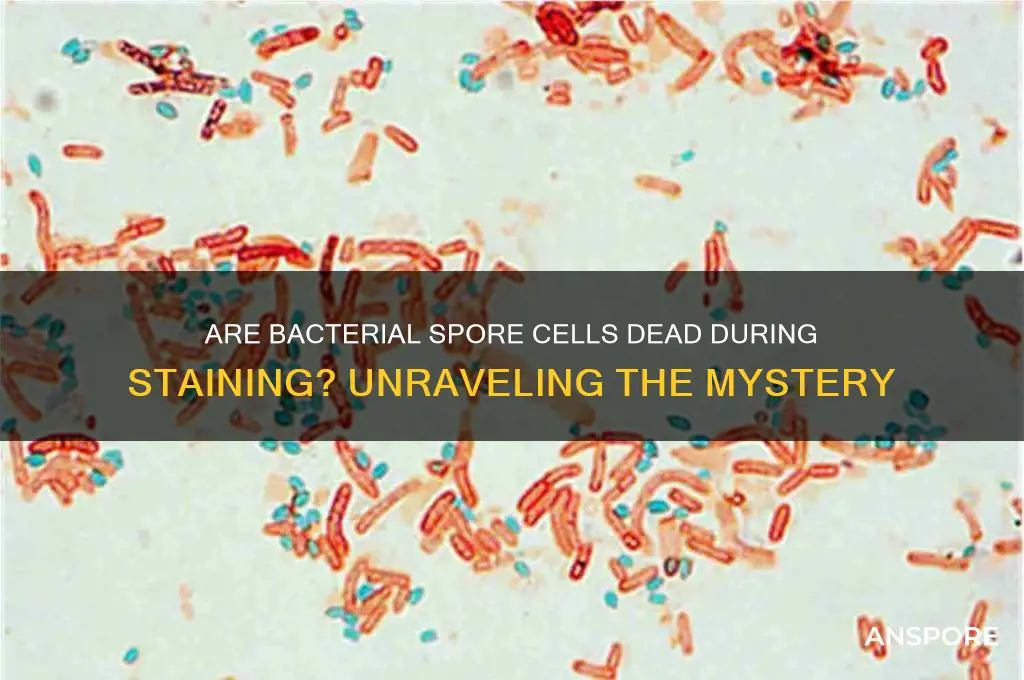
Bacterial spores are remarkably resilient structures, capable of surviving extreme conditions that would destroy vegetative cells. However, this durability poses a challenge in staining techniques, as spores often resist conventional dyes. The Schaeffer-Fulton method emerges as a reliable solution, employing a combination of heat and specific stains to differentiate spores from vegetative cells. This technique hinges on the spore’s impermeable nature, which allows it to retain the primary stain even after decolorization, while the vegetative cells lose their color.
To execute the Schaeffer-Fulton stain, begin by preparing a bacterial smear on a clean slide and allowing it to air-dry. Fix the smear by gently passing it through a flame 2–3 times, ensuring the heat does not char the sample. Next, flood the slide with malachite green, a primary stain, and heat-fix again for 5 minutes. This step is critical, as the heat drives the stain into the spore’s resistant coat. After cooling, decolorize the slide with water for 30–60 seconds, followed by a counterstain with safranin for 2–3 minutes. Rinse, blot dry, and examine under a microscope. Spores will appear green, while vegetative cells will be pink or red, providing a clear distinction.
One of the key advantages of the Schaeffer-Fulton method is its specificity. Unlike vegetative cells, spores are not metabolically active during staining, yet they retain the malachite green due to their unique structure. This raises the question: are the cells dead in bacterial spore staining? While spores are dormant and metabolically inactive, they are not dead. They remain viable, capable of germinating under favorable conditions. The staining process does not kill them but rather highlights their distinct characteristics.
A comparative analysis of spore staining techniques reveals why Schaeffer-Fulton stands out. Alternative methods, such as the Dorner method, use cold staining with chloramine-T to enhance malachite green penetration. However, Schaeffer-Fulton’s heat-based approach is more straightforward and widely applicable, especially for beginners. Its simplicity and reliability make it a staple in microbiology labs, particularly for identifying spore-forming bacteria like *Bacillus* and *Clostridium*.
In practical applications, understanding the nuances of spore staining is essential. For instance, over-heating the slide can damage the sample, while insufficient heat may result in poor staining. Additionally, the choice of counterstain can influence contrast and clarity. Safranin is commonly used, but other dyes like fuchsine can be substituted based on availability. By mastering the Schaeffer-Fulton technique, microbiologists can accurately differentiate spores from vegetative cells, aiding in diagnosis, research, and quality control in industries like food and pharmaceuticals.
Mold Spores vs. Dust Mites: Which is Smaller in Size?
You may want to see also

Spore Dormancy State: Metabolic inactivity and resistance mechanisms in bacterial spores
Bacterial spores are masters of survival, entering a dormant state that defies easy categorization as "alive" or "dead." This spore dormancy state is characterized by profound metabolic inactivity, a key mechanism for enduring harsh environmental conditions. During sporulation, the bacterial cell differentiates into a highly resistant spore form, shedding most of its water content and replacing it with protective molecules like dipicolinic acid (DPA). This transformation results in a metabolic slowdown so extreme that spores can remain viable for centuries, even millennia, under conditions that would swiftly destroy vegetative cells.
The resistance mechanisms of bacterial spores are multifaceted and remarkably efficient. The spore’s outer layers, including the spore coat and exosporium, act as physical barriers against desiccation, heat, and chemicals. The core, where the bacterial DNA resides, is dehydrated and stabilized by DPA, which chelates calcium ions to form a lattice that protects DNA from damage. Additionally, spores possess DNA repair enzymes that activate upon germination, ensuring genetic integrity despite prolonged dormancy. These adaptations collectively render spores resistant to UV radiation, extreme temperatures, and antimicrobial agents, making them a formidable challenge in sterilization processes.
To illustrate the practical implications, consider the healthcare and food industries, where spore-forming bacteria like *Clostridium botulinum* and *Bacillus cereus* pose significant risks. Standard disinfection methods, such as alcohol-based sanitizers or boiling water, are ineffective against spores. Complete sterilization requires more aggressive measures, such as autoclaving at 121°C for 15–30 minutes or the use of sporicidal chemicals like hydrogen peroxide or peracetic acid. Even then, spores’ resistance necessitates precise control of time, temperature, and concentration to ensure efficacy.
A critical takeaway is that the dormancy state of bacterial spores is not a passive condition but an active, highly regulated process. Spores are not dead; they are metabolically inactive yet retain the potential for revival under favorable conditions. This distinction is crucial for understanding their resilience and designing effective strategies to combat them. For instance, in laboratory settings, researchers use specific germination triggers, such as nutrient availability and temperature shifts, to reactivate spores for study. Similarly, in industrial applications, understanding spore resistance helps optimize sterilization protocols to ensure safety and efficacy.
In summary, the spore dormancy state is a testament to bacterial adaptability, blending metabolic inactivity with robust resistance mechanisms. While spores may appear lifeless, their ability to withstand extreme conditions and revive underscores their unique biological status. Recognizing this duality is essential for addressing the challenges posed by spore-forming bacteria in various fields, from medicine to food safety. By targeting their resistance mechanisms and leveraging their reactivation processes, we can develop more effective strategies to control and utilize these remarkable microbial survivors.
Are Psilocybin Mushroom Spores Illegal? Understanding the Legal Landscape
You may want to see also
Explore related products

Spore Viability Tests: Assessing if spores are alive or dead post-staining
Bacterial spores are renowned for their resilience, capable of withstanding extreme conditions such as heat, desiccation, and chemicals. However, determining whether spores remain viable after staining procedures is crucial for both research and industrial applications. Spore viability tests serve this purpose by distinguishing between live and dead spores post-staining, ensuring accurate interpretation of results and maintaining the integrity of downstream processes.
One widely adopted method for assessing spore viability is the germination assay. This technique involves exposing stained spores to conditions that promote germination, such as nutrient-rich media and optimal temperature (typically 37°C). Live spores will germinate, evidenced by changes in morphology, such as the emergence of vegetative cells. In contrast, dead spores remain dormant. For precise quantification, researchers often use a direct viable count (DVC), where spores are plated on agar and incubated for 12–24 hours. The number of colonies formed correlates directly with the number of viable spores. A key advantage of this method is its simplicity and reliability, though it requires careful control of incubation conditions to avoid false negatives.
Another approach is the fluorescent viability staining technique, which employs dyes like propidium iodide (PI) or SYTO 9. PI penetrates compromised cell membranes, staining dead spores red, while SYTO 9 labels all spores green. When used in tandem, live spores appear green, and dead spores appear red or orange. This method offers rapid results, often within 15–30 minutes, and is particularly useful for high-throughput screening. However, it requires specialized equipment like a fluorescence microscope and may yield false positives if the staining solution damages live spores. Dosage is critical here; typically, a 1:1 ratio of SYTO 9 to PI is recommended, with a final concentration of 5 μM for each dye.
For industrial applications, such as food preservation or pharmaceutical manufacturing, heat shock treatment followed by viability testing is often employed. Spores are exposed to sublethal temperatures (e.g., 80°C for 10 minutes) to induce germination, and their viability is then assessed via plating or microscopy. This method mimics real-world conditions and ensures that only fully viable spores are detected. A cautionary note: over-exposure to heat can kill live spores, so precise timing and temperature control are essential.
In conclusion, spore viability tests are indispensable tools for verifying the survival status of spores post-staining. Whether through germination assays, fluorescent staining, or heat shock treatments, each method offers unique advantages and requires careful execution. By selecting the appropriate technique and adhering to specific protocols, researchers and industry professionals can ensure accurate and reliable results, ultimately advancing their work in microbiology and beyond.
Understanding Spore Prints: A Beginner's Guide to Mushroom Identification
You may want to see also

Spore Germination Factors: Conditions required to reactivate dormant bacterial spores
Bacterial spores are renowned for their resilience, capable of surviving extreme conditions that would destroy most life forms. However, these dormant structures are not dead; they are metabolically inactive, waiting for the right environmental cues to reactivate. Understanding the factors that trigger spore germination is crucial for both harnessing their potential in biotechnology and controlling their proliferation in food safety and healthcare.
Triggering Germination: Key Environmental Cues
Spore germination begins with the perception of specific environmental signals. Nutrient availability, particularly L-alanine, glycine, and inosine, acts as a primary stimulant. For instance, *Bacillus subtilis* spores require a concentration of 10–20 mM L-alanine to initiate germination. Temperature also plays a critical role; most bacterial spores germinate optimally between 25°C and 37°C, though some thermophilic species, like *Geobacillus stearothermophilus*, require temperatures above 50°C. pH levels between 7 and 8 are generally favorable, though spores can tolerate a range of 5 to 9. Additionally, water activity (aw) must be above 0.9 for germination to occur, as spores remain dormant in dry conditions.
The Role of Physical and Chemical Stressors
While optimal conditions are essential, certain stressors can paradoxically enhance germination. Mild heat shock, such as exposure to 70°C for 30 minutes, can prime spores for faster reactivation. Similarly, sublethal doses of UV radiation or oxidizing agents like hydrogen peroxide (0.1–1 mM) can stimulate germination by damaging the spore coat, making it more permeable to germinants. However, excessive stress can lead to spore inactivation, underscoring the delicate balance required for successful reawakening.
Practical Applications and Cautions
In industrial settings, controlling spore germination is vital for processes like enzyme production and probiotic activation. For example, adding 50 mM inosine to a culture medium can significantly accelerate spore germination in bioreactors. Conversely, in food preservation, preventing germination is paramount. Techniques like pasteurization (72°C for 15 seconds) or the use of germinant inhibitors, such as D-alanine, can effectively suppress spore reactivation. It’s critical to monitor these conditions closely, as even minor deviations can lead to unintended germination or incomplete inactivation.
Comparative Insights: Species-Specific Requirements
Different bacterial species exhibit unique germination requirements, reflecting their ecological niches. For instance, *Clostridium botulinum* spores require specific sugars like glucose or fructose, while *Bacillus anthracis* responds to calcium-dipicolinic acid complexes. This diversity highlights the importance of tailored approaches in both research and application. Understanding these species-specific triggers not only aids in controlling pathogenic spores but also informs the development of spore-based technologies, such as targeted drug delivery systems.
By mastering the conditions that reactivate dormant bacterial spores, we can leverage their durability for innovation while mitigating their risks in critical areas like food safety and medicine. Whether in the lab or the field, precision in manipulating these factors is key to unlocking the potential—or neutralizing the threat—of bacterial spores.
Understanding Isolated Spore Syringes: A Beginner's Guide to Mushroom Cultivation
You may want to see also
Frequently asked questions
No, the cells are not necessarily dead during bacterial spore staining. The process primarily targets and visualizes spores, which are dormant, highly resistant structures produced by certain bacteria. The vegetative cells may still be viable.
The staining process itself, such as the Schaeffer-Fulton or Dorner methods, does not kill the bacterial cells. However, the heat fixation step involved in some staining procedures may kill or inactivate the vegetative cells, while spores remain viable due to their resistance.
Yes, bacterial spores can still be stained even if the vegetative cells are dead. Spores retain their structural integrity and staining properties, allowing them to be visualized regardless of the viability of the surrounding cells.
Determining cell viability after spore staining requires additional tests, such as viability staining (e.g., using dyes like propidium iodide) or culturing the sample. Spore staining alone does not provide information about cell viability.