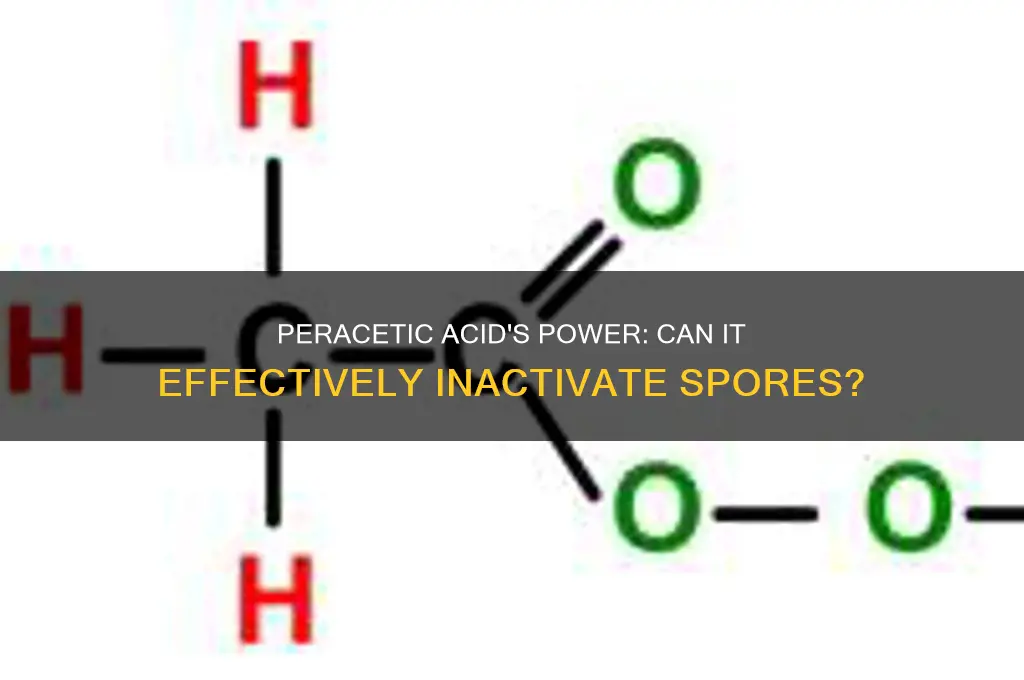
Peracetic acid (PAA) is a powerful oxidizing agent widely recognized for its efficacy in disinfection and sterilization processes across various industries, including healthcare, food processing, and water treatment. Its ability to inactivate a broad spectrum of microorganisms, including bacteria, viruses, and fungi, has been well-documented. However, the question of whether peracetic acid can effectively inactivate spores—highly resistant forms of certain bacteria, such as *Clostridium difficile* and *Bacillus* species—remains a critical area of investigation. Spores are notoriously resilient due to their robust outer coat and low metabolic activity, making them challenging to eradicate. Research indicates that peracetic acid can indeed inactivate spores, but the effectiveness depends on factors such as concentration, exposure time, temperature, and the specific spore species. Understanding the mechanisms and conditions under which PAA can successfully neutralize spores is essential for optimizing its use in sterilization protocols and ensuring public health and safety.
| Characteristics | Values |
|---|---|
| Effectiveness | Peracetic acid (PAA) is highly effective in inactivating spores, including bacterial spores like those of Clostridium difficile and Bacillus species. |
| Mechanism of Action | PAA disrupts spore structure by oxidizing proteins, lipids, and nucleic acids, leading to spore coat damage and core inactivation. |
| Concentration Required | Typically, concentrations of 0.1% to 1% PAA are effective for spore inactivation, depending on contact time and spore type. |
| Contact Time | Longer contact times (e.g., 10–30 minutes) enhance spore inactivation efficacy, though shorter times may suffice at higher concentrations. |
| Temperature Influence | Higher temperatures (e.g., 20–40°C) improve PAA's sporicidal activity by increasing reaction kinetics. |
| pH Dependence | PAA is most effective in slightly acidic to neutral pH conditions (pH 6–8), as extreme pH reduces its stability and activity. |
| Applications | Widely used in healthcare, food processing, and water treatment for disinfection and sterilization due to its broad-spectrum antimicrobial activity. |
| Resistance | Spores are generally more resistant than vegetative cells, but PAA remains effective against most spore-forming bacteria under proper conditions. |
| Safety | PAA is corrosive and requires careful handling; it decomposes into acetic acid, oxygen, and water, making it environmentally friendly. |
| Regulations | Approved by regulatory bodies (e.g., EPA, FDA) for use in various industries due to its efficacy and safety profile. |
Explore related products
What You'll Learn
- Effectiveness of peracetic acid concentrations on spore inactivation
- Role of exposure time in spore inactivation by peracetic acid
- Impact of temperature on peracetic acid’s spore inactivation ability
- Comparison of peracetic acid with other spore inactivating agents
- Mechanisms of spore inactivation by peracetic acid

Effectiveness of peracetic acid concentrations on spore inactivation
Peracetic acid (PAA) is a potent oxidizing agent widely recognized for its antimicrobial properties, but its efficacy against spores—particularly bacterial spores—is a critical area of interest in disinfection and sterilization processes. Spores are notoriously resistant to chemical and physical agents due to their robust structure, which includes a thick protein coat and a highly impermeable outer layer. However, studies have shown that PAA can indeed inactivate spores, though its effectiveness is highly dependent on concentration, contact time, and environmental conditions. For instance, concentrations of 0.3% to 1.0% PAA have been found to achieve significant spore reduction within 10 to 30 minutes, making it a viable option for industries requiring high-level disinfection, such as healthcare and food processing.
When considering the practical application of PAA for spore inactivation, it is essential to balance efficacy with safety and material compatibility. Higher concentrations of PAA (e.g., 2% or greater) can achieve faster and more complete spore inactivation but may pose risks to surfaces, equipment, and personnel due to its corrosive nature. For example, in healthcare settings, 0.5% PAA solutions are often used for instrument disinfection, as they strike a balance between spore-killing power and material preservation. In contrast, lower concentrations (0.2% to 0.3%) may be sufficient for routine surface disinfection in food processing facilities, where spore contamination is less critical but still a concern. Always follow manufacturer guidelines and conduct compatibility testing to avoid damage to sensitive materials.
A comparative analysis of PAA with other sporocidal agents, such as hydrogen peroxide or formaldehyde, highlights its advantages and limitations. Unlike formaldehyde, PAA does not leave harmful residues and decomposes into non-toxic byproducts (acetic acid, oxygen, and water), making it safer for use in food and pharmaceutical environments. However, PAA’s efficacy can be compromised in the presence of organic matter, which underscores the importance of pre-cleaning surfaces before disinfection. Hydrogen peroxide, while also effective against spores, often requires longer contact times or higher temperatures to match PAA’s performance at optimal concentrations. This makes PAA a more practical choice in scenarios where rapid disinfection is necessary.
To maximize the effectiveness of PAA in spore inactivation, follow these steps: first, ensure the target surface or material is free of organic debris through thorough cleaning. Second, select an appropriate PAA concentration based on the level of contamination and the desired contact time—for example, 0.5% PAA for 20 minutes is commonly recommended for high-level disinfection. Third, monitor environmental factors such as temperature and pH, as PAA’s stability and activity can be affected by these conditions. Finally, verify the disinfection process through spore testing, particularly in critical applications like medical device sterilization. By adhering to these guidelines, PAA can be a reliable and efficient tool for spore inactivation across various industries.
Creating Spore Prints on Paper Towels: A Simple DIY Guide
You may want to see also

Role of exposure time in spore inactivation by peracetic acid
Peracetic acid (PAA) is a potent oxidizing agent known for its efficacy against a wide range of microorganisms, including bacterial spores. However, the success of spore inactivation hinges critically on exposure time. Studies show that even at high concentrations, PAA requires sufficient contact duration to penetrate the spore’s resilient coat and disrupt its core structures. For instance, *Bacillus subtilis* spores, commonly used as surrogates for *Clostridium difficile*, exhibit significant reduction in viability after 10 minutes of exposure to 200 ppm PAA, but complete inactivation may demand up to 30 minutes depending on environmental conditions.
To maximize spore inactivation, follow these steps: first, ensure PAA concentration aligns with the target spore type—typically 100–500 ppm for bacterial spores. Second, maintain a consistent temperature, as higher temperatures (e.g., 25°C vs. 10°C) can reduce required exposure time by accelerating PAA’s oxidative action. Third, monitor pH levels; PAA is most effective in neutral to slightly acidic conditions (pH 6–8). Finally, agitate the solution to ensure uniform spore contact with PAA, as stagnant conditions may leave spores inadequately exposed.
A comparative analysis reveals that exposure time is more influential than concentration in spore inactivation. For example, increasing PAA from 200 ppm to 400 ppm may halve the required exposure time but only marginally improves efficacy beyond a certain threshold. Conversely, extending exposure time from 10 to 20 minutes at a fixed concentration (e.g., 200 ppm) consistently yields higher log reductions in spore counts. This underscores the importance of prioritizing exposure duration over concentration adjustments in practical applications.
Practical tips for optimizing exposure time include pre-treating surfaces or equipment to remove organic matter, which can shield spores from PAA. Additionally, use automated systems with timers to ensure precise exposure durations, particularly in industrial settings like food processing or healthcare disinfection. For small-scale applications, such as laboratory experiments, employ stopwatches and standardized protocols to eliminate variability. Remember, longer exposure times are not always feasible due to PAA’s instability, so balance duration with concentration to achieve effective spore inactivation without compromising efficiency.
From Barracks to Beliefs: Can Military Cities Transform into Religious Hubs?
You may want to see also

Impact of temperature on peracetic acid’s spore inactivation ability
Peracetic acid (PAA) is a potent disinfectant known for its efficacy against a wide range of microorganisms, including spores. However, its spore inactivation ability is not constant; temperature plays a critical role in determining its effectiveness. At lower temperatures (e.g., 10–20°C), PAA’s spore inactivation efficiency decreases significantly due to reduced chemical reactivity and slower diffusion rates. For instance, studies show that at 15°C, a 200 ppm PAA solution requires up to 60 minutes to achieve complete spore inactivation, whereas at 30°C, the same concentration can achieve the same result in under 15 minutes. This highlights the importance of temperature optimization in practical applications, such as food processing or medical device sterilization.
To maximize PAA’s spore inactivation ability, temperature control must be paired with appropriate dosage and contact time. At elevated temperatures (40–50°C), PAA’s efficacy increases dramatically, but caution is necessary to avoid material degradation or safety risks. For example, in the dairy industry, PAA solutions at 400 ppm applied at 45°C for 10 minutes are commonly used to sanitize equipment contaminated with *Bacillus* spores. However, temperatures above 60°C can accelerate PAA decomposition, reducing its active concentration and effectiveness. Thus, maintaining a temperature range of 35–50°C is recommended for optimal spore inactivation without compromising PAA stability.
Comparatively, PAA outperforms other disinfectants like hydrogen peroxide or chlorine at moderate temperatures, particularly against spore-forming bacteria. While chlorine’s efficacy drops significantly below 20°C, PAA retains a degree of activity, making it a more reliable choice in cooler environments. However, unlike heat sterilization methods (e.g., autoclaving at 121°C), PAA’s temperature dependence limits its use in high-temperature applications. Practitioners must therefore balance temperature, concentration, and exposure time to achieve consistent spore inactivation, especially in industries where spores pose a persistent challenge, such as brewing or pharmaceutical manufacturing.
Practical tips for leveraging temperature to enhance PAA’s spore inactivation ability include preheating surfaces or solutions to 35–40°C before application and ensuring uniform distribution to avoid cold spots. For example, in water treatment systems, circulating PAA solutions at 40°C can effectively target *Clostridium* spores, which are notoriously resistant. Additionally, monitoring PAA concentration post-application is crucial, as higher temperatures can increase evaporation or decomposition rates. By integrating temperature control into PAA protocols, industries can achieve reliable spore inactivation while minimizing chemical waste and operational costs.
Can Your Vacuum Harbor Ringworm Spores? Uncovering the Hidden Risks
You may want to see also
Explore related products
$35.53 $39.11

Comparison of peracetic acid with other spore inactivating agents
Peracetic acid (PAA) stands out as a potent spore inactivating agent, but its efficacy and practicality must be weighed against alternatives like hydrogen peroxide, formaldehyde, and chlorine dioxide. Each agent has distinct properties, application methods, and limitations, making the choice context-dependent. For instance, PAA’s rapid degradation into non-toxic byproducts (acetic acid, oxygen, and water) offers an environmental advantage over formaldehyde, which leaves residues requiring careful disposal. However, formaldehyde’s superior penetration through organic matter makes it more effective in certain scenarios, such as sterilizing medical devices with complex geometries.
Dosage and contact time are critical factors in comparing these agents. PAA typically requires concentrations of 0.1–0.35% (w/v) with exposure times of 10–30 minutes to inactivate spores, depending on the species and application. In contrast, hydrogen peroxide, often used in vaporized form (VHP), demands higher concentrations (35–59%) and longer exposure times (1–6 hours) but excels in large-scale decontamination, such as in pharmaceutical cleanrooms. Chlorine dioxide, another strong oxidizer, is effective at lower concentrations (3–5 ppm) but is less stable and requires careful handling due to its corrosive nature. This variability underscores the need to match the agent to the specific requirements of the task.
Practical considerations further differentiate these agents. PAA’s compatibility with most materials, including metals and plastics, makes it versatile for disinfecting equipment in food processing and healthcare settings. However, its limited stability in solution necessitates frequent monitoring and replenishment, unlike chlorine dioxide, which can be generated on-site but poses risks of gas release. Hydrogen peroxide’s broad-spectrum efficacy is tempered by its potential to degrade certain materials, such as rubber and textiles, requiring careful selection of application methods. These trade-offs highlight the importance of balancing efficacy with operational feasibility.
Instructively, selecting the right spore inactivating agent involves a step-by-step evaluation: identify the target spore species, assess the application environment (e.g., surface type, scale, and organic load), and consider logistical constraints like storage, handling, and regulatory compliance. For example, in water treatment, PAA’s rapid action and low environmental impact make it a preferred choice over chlorine dioxide, which can produce harmful byproducts like chlorite ions. Conversely, in high-throughput sterilization processes, VHP’s ability to penetrate hard-to-reach areas may outweigh its longer processing times.
Ultimately, while peracetic acid offers a compelling combination of efficacy, safety, and environmental friendliness, it is not a one-size-fits-all solution. Its comparison with other agents reveals a spectrum of options, each with unique strengths and limitations. The key takeaway is to tailor the choice to the specific demands of the application, ensuring both effective spore inactivation and operational practicality. This nuanced approach ensures optimal outcomes in diverse settings, from healthcare to industrial manufacturing.
Are Spore Syringes Legal in Canada? Understanding the Current Laws
You may want to see also

Mechanisms of spore inactivation by peracetic acid
Peracetic acid (PAA) is a potent oxidizing agent known for its efficacy against a wide range of microorganisms, including bacterial spores. Its ability to inactivate spores is particularly significant in industries such as food processing, healthcare, and water treatment, where spore contamination poses serious risks. The mechanisms by which PAA achieves spore inactivation are multifaceted, involving both physical and chemical interactions with the spore’s structure and components.
One primary mechanism is the disruption of the spore’s protective coat and cortex. Spores are encased in a multilayered structure designed to withstand harsh conditions. PAA penetrates these layers, oxidizing proteins and lipids that maintain the integrity of the coat and cortex. This degradation weakens the spore’s defenses, making it more susceptible to further damage. For example, studies have shown that PAA at concentrations of 200–500 ppm can effectively compromise the spore’s outer layers within minutes, depending on the species and exposure time.
Another critical mechanism is the inactivation of spore enzymes and DNA. PAA’s oxidizing properties allow it to react with essential enzymes involved in spore germination and outgrowth, rendering them nonfunctional. Additionally, PAA can directly damage the spore’s DNA by oxidizing nucleotides, preventing replication and repair processes. This dual attack on both enzymatic activity and genetic material ensures that even if a spore survives initial exposure, it is unlikely to regain viability. Practical applications often involve PAA concentrations of 1,000–2,000 ppm for thorough spore inactivation, particularly in high-risk environments like pharmaceutical manufacturing.
Comparatively, PAA’s efficacy against spores is often contrasted with other disinfectants like hydrogen peroxide or chlorine. Unlike these agents, PAA remains active in the presence of organic matter, making it more reliable in real-world scenarios where contaminants are common. Its low pH (typically 2–4) also enhances its antimicrobial activity by facilitating spore coat penetration. However, users must exercise caution, as PAA’s corrosive nature requires proper handling and material compatibility checks, especially in industrial settings.
In summary, the mechanisms of spore inactivation by peracetic acid involve coat and cortex disruption, enzyme inactivation, and DNA damage. Its effectiveness is dose-dependent, with higher concentrations and longer exposure times yielding better results. For practical use, combining PAA with other sanitizing agents or physical treatments (e.g., heat) can enhance spore inactivation, particularly for highly resistant species like *Clostridium sporogenes*. Always follow manufacturer guidelines and safety protocols to maximize efficacy while minimizing risks.
How Mold Spores Travel: Can They Contaminate Other Objects?
You may want to see also
Frequently asked questions
Yes, peracetic acid is highly effective at inactivating spores, including those of bacteria and fungi, due to its strong oxidizing properties.
Typically, concentrations ranging from 200 to 1,000 ppm (parts per million) are effective for spore inactivation, depending on the exposure time and spore type.
The time required varies, but generally, exposure times of 10 to 30 minutes are sufficient for effective spore inactivation at recommended concentrations.
Yes, peracetic acid is widely used in food processing, healthcare, and water treatment for spore inactivation, but proper handling and safety precautions are essential due to its corrosive nature.