Psilocybin mushrooms, also known as magic mushrooms, are currently classified as Schedule I drugs under the United Nations 1971 Convention on Psychotropic Substances. Schedule I drugs are defined as drugs with a high potential for abuse and no recognized medical uses. Psilocybin mushrooms have been used medicinally and religiously in various cultures throughout history, and some researchers argue that they have a significantly lower potential for abuse than other Schedule I drugs. The legal status of psilocybin mushrooms varies worldwide, with some jurisdictions criminalizing their possession and sale, while others, like the District of Columbia and Colorado, have moved towards decriminalization and regulated use.
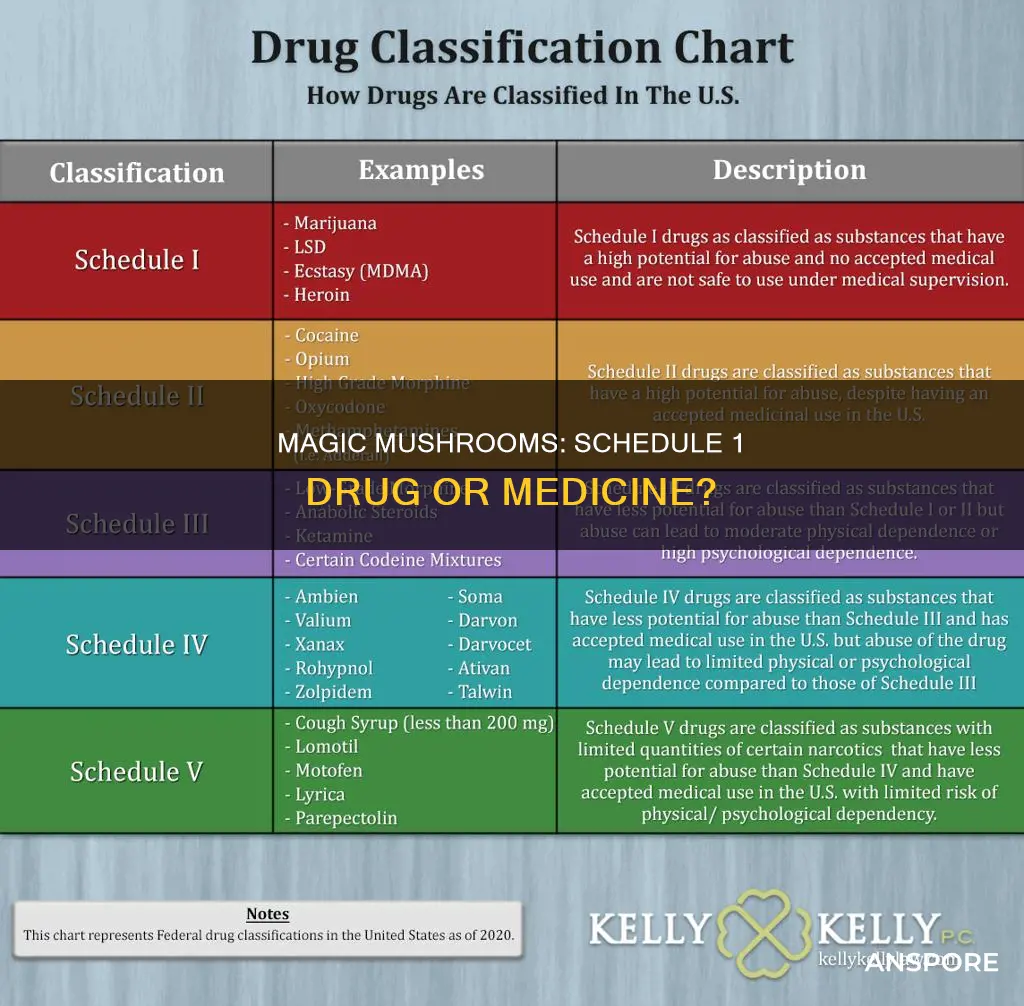
| Characteristics | Values |
|---|---|
| Type of Drug | Psilocybin and psilocin are the drugs found in magic mushrooms. |
| Schedule | Psilocybin mushrooms are Schedule I drugs under the United Nations 1971 Convention on Psychotropic Substances. |
| Schedule I Definition | Schedule I drugs are defined as drugs with a high potential for abuse and no recognized medical uses. |
| Legality | The legal status of psilocybin mushrooms varies worldwide, with many countries having some level of regulation or prohibition. |
| Legality in the US | Psilocybin and psilocin are regulated by the Controlled Substances Act and are considered Schedule I drugs in the US. However, there is ambiguity in the legal status, as some US states have decriminalized or legalized psilocybin mushrooms for medical or therapeutic use. |
| Legality in Canada | In January 2023, the Canadian province of Alberta allowed the use of psilocybin for medicinal purposes in drug-assisted psychotherapy. |
| Legality in Australia | In February 2023, Australia approved the use of psilocybin in prescription medications for the treatment of PTSD and treatment-resistant depression. |
| Potential for Abuse | Studies suggest that psilocybin has a relatively low risk of abuse compared to other drugs. |
| Safety | Psilocybin has a lower potential for harm to users and society, and there is no known overdose level. |
| Reclassification Suggestions | Researchers suggest that if psilocybin clears phase III clinical trials, it could be reclassified as a Schedule IV drug with tighter control. |
Explore related products
What You'll Learn
- Psilocybin mushrooms are Schedule I drugs, deemed to have a high potential for abuse and no accepted medical use
- The mushrooms have a long history of medicinal and religious use in many cultures, and a lower potential for abuse than other Schedule I drugs
- The legal status of psilocybin mushrooms varies worldwide, with some countries and US states decriminalising or regulating their use
- In some jurisdictions, the cultivation or possession of psilocybin mushroom spores is prohibited, while the spores themselves are often legal
- Researchers suggest that psilocybin mushrooms could be reclassified as Schedule IV drugs, similar to prescription sleep aids, if clinical trials are successful

Psilocybin mushrooms are Schedule I drugs, deemed to have a high potential for abuse and no accepted medical use
Psilocybin mushrooms, often referred to as "magic mushrooms", are classified as Schedule I drugs in the United States. Schedule I drugs are defined by the United States Drug Enforcement Agency (DEA) as substances with a high potential for abuse and no currently accepted medical use in the country. Psilocybin mushrooms are also listed as Schedule I drugs under the United Nations 1971 Convention on Psychotropic Substances, which requires its members to prohibit the substance.
The classification of psilocybin mushrooms as Schedule I drugs means that they are considered to have a high potential for abuse and no accepted medical use. This classification is based on the Controlled Substances Act (CSA), which categorises drugs into five schedules based on their potential for abuse and medical utility. Schedule I drugs are the only category of drugs that cannot be prescribed by medical professionals. Other examples of Schedule I drugs include heroin, gamma-hydroxybutyric acid (GHB), lysergic acid diethylamide (LSD), and marijuana.
The classification of psilocybin mushrooms as Schedule I drugs has been a subject of debate due to their historical use in medicinal and religious contexts by various cultures. Researchers at Johns Hopkins University have suggested that psilocybin mushrooms should be reclassified as Schedule IV drugs, which are substances with accepted medical use but a lower potential for abuse compared to Schedule I drugs. These researchers highlight the relatively low risk and low abuse potential of psilocybin mushrooms, with animal and human studies indicating limited usage and reduced harm compared to other substances.
Despite the ongoing debate and evolving legal landscape, the classification of psilocybin mushrooms as Schedule I drugs persists in many jurisdictions. This classification carries significant legal implications, influencing the regulation, prohibition, and enforcement approaches associated with these substances.
Mushrooms: Nature's Rare Delicacy
You may want to see also

The mushrooms have a long history of medicinal and religious use in many cultures, and a lower potential for abuse than other Schedule I drugs
Psilocybin mushrooms, commonly known as "magic mushrooms", are listed as Schedule I drugs under the United Nations 1971 Convention on Psychotropic Substances. Schedule I drugs are defined as drugs with a high potential for abuse and no recognized medical uses. However, the potential for abuse and the lack of therapeutic benefit associated with psilocybin mushrooms are debated.
Magic mushrooms have a long history of medicinal and religious use in many cultures. The use of psychoactive substances in religious rites and rituals dates back thousands of years. For example, archaeological evidence from a gravesite in northern Iraq suggests that prehistoric ancestors consumed psychoactive substances found in nature. Additionally, the ancient Hindu text, the Rig Veda, describes a drink called soma, which some historians believe may have contained psilocybin. In ancient times, the Greek physician Hippocrates classified the amadou mushroom as a potent anti-inflammatory and wound-healing agent. The alchemist Tao Hongjing, from the 5th century, described several medicinal mushrooms, including ling zhi and zhu ling, which were reportedly used by Shennong many centuries earlier.
In modern times, the therapeutic effects of psilocybin mushrooms have been explored in the treatment of conditions such as PTSD, addiction, and existential distress in terminal cancer patients. Research is ongoing, but psilocybin may show promise in helping to treat or manage these and other conditions. Psilocybin mushrooms also have a low risk of addiction, with some sources stating that they have a significantly lower potential for abuse than other Schedule I drugs.
Despite their potential medicinal benefits, magic mushrooms can cause adverse side effects, such as visual flashbacks and traumatic recalls of intensely upsetting experiences, known as hallucinogen-persisting perception disorder, which is rare. Additionally, there is a risk of accidental poisoning from consuming poisonous mushrooms. Due to the potential risks associated with psilocybin mushrooms, their legal status varies worldwide, with some countries and jurisdictions prohibiting or regulating their use.
Mushrooms: Hallucinogens or Not?
You may want to see also

The legal status of psilocybin mushrooms varies worldwide, with some countries and US states decriminalising or regulating their use
Psilocybin and psilocin, the active substances in psilocybin mushrooms, are listed as Schedule I drugs under the United Nations 1971 Convention on Psychotropic Substances. Schedule I drugs are defined as drugs with a high potential for abuse and no recognised medical uses. However, psilocybin mushrooms have been used medicinally and religiously in numerous cultures throughout history, and their potential for abuse is significantly lower than that of other Schedule I drugs.
While the UN convention requires its members to prohibit psilocybin, the mushrooms themselves were not specifically included in the convention due to pressure from the Mexican government. As a result, the legal status of psilocybin mushrooms varies worldwide, with some countries and US states decriminalising or regulating their use. Many countries have some level of regulation or prohibition of psilocybin mushrooms, such as the US Psychotropic Substances Act, the UK Misuse of Drugs Act 1971, and the Canadian Controlled Drugs and Substances Act.
In some jurisdictions, psilocybin mushroom spores are legal to sell and possess because they do not contain psilocybin or psilocin. However, jurisdictions like Germany, California, Georgia, and Idaho have specifically prohibited the sale and possession of these spores. Cultivation of psilocybin mushrooms is generally considered drug manufacture and is severely penalised, though New Mexico has ruled that it does not qualify as manufacturing a controlled substance.
There is a growing trend of localities, particularly in North America, revising their legal frameworks regarding psychedelics. For example, Oregon and Colorado have legalised specific psychedelics, and cities like Oakland and Washington, DC, have made enforcing psychedelics' illegality a low priority for law enforcement. In November 2020, voters in Oregon passed a ballot initiative legalising "magic mushrooms" for mental health treatment in supervised settings. The District of Columbia also passed a similar initiative in 2020, allowing for the possession and non-profit gifting or distribution of psilocybin mushrooms.
In October 2022, the Canadian province of Alberta announced it would regulate and allow the use of psilocybin and other substances for medicinal purposes in drug-assisted psychotherapy. This regulation came into effect in January 2023. Australia has also approved the use of psilocybin and MDMA in prescription medications for treating PTSD and treatment-resistant depression as of February 2023. These changes reflect the growing scientific research highlighting the therapeutic and medicinal potential of psychedelics, which has garnered increasing legal and political support.
Stir-Frying Mushrooms: Tips and Tricks for Success
You may want to see also
Explore related products

In some jurisdictions, the cultivation or possession of psilocybin mushroom spores is prohibited, while the spores themselves are often legal
Psilocybin and psilocin, the primary psychoactive compounds found in psilocybin mushrooms, are listed as Schedule I drugs under the United Nations 1971 Convention on Psychotropic Substances. Schedule I drugs are defined as drugs with a high potential for abuse and no recognised medical uses. Psilocybin mushrooms, however, have had numerous medicinal and religious uses in several cultures throughout history and have a significantly lower potential for abuse than other Schedule I drugs.
The legal status of unauthorised actions with psilocybin mushrooms varies worldwide, with many national, state, and provincial drug laws exhibiting ambiguity and selective enforcement. Most US state courts consider the mushroom a "container" of illicit drugs, thus deeming it illegal. However, a loophole exists regarding the spores of psilocybin mushrooms, which do not contain psilocybin or psilocin. As a result, the possession, sale, and cultivation of spores are legal in many areas, while the same actions with the mushrooms are prohibited.
Some jurisdictions, such as California, Georgia, and Idaho in the United States, and Germany, have specifically prohibited the sale and possession of psilocybin mushroom spores. In these places, the cultivation of psilocybin mushrooms is often considered drug manufacture and can result in severe penalties. However, a few countries and one US state, New Mexico, have ruled that growing psilocybin mushrooms does not constitute "manufacturing" a controlled substance. This discrepancy has led to an underground economy and social networks supporting the illicit trade of spores and cultivation materials.
While the United Nations Convention on Psychotropic Substances requires its members to prohibit psilocybin, the mushrooms containing the drug were not specifically included in the convention due to pressure from the Mexican government. This has resulted in varying national drug laws, with some countries regulating or prohibiting psilocybin mushrooms through legislation such as the US Psychotropic Substances Act, the UK Misuse of Drugs Act 1971, and the Canadian Controlled Drugs and Substances Act. Despite the legal complexities, the possession and use of psilocybin are generally prohibited under almost all circumstances, attracting severe legal penalties.
Mushroom Magic: Unlocking Savory Sauteed Flavors
You may want to see also

Researchers suggest that psilocybin mushrooms could be reclassified as Schedule IV drugs, similar to prescription sleep aids, if clinical trials are successful
Psilocybin mushrooms, often referred to as "magic mushrooms", are currently classified as Schedule I drugs under the United Nations 1971 Convention on Psychotropic Substances. Schedule I drugs are defined as drugs with a high potential for abuse and no recognised medical uses. Psilocybin mushrooms have had numerous medicinal and religious uses in dozens of cultures throughout history and have a significantly lower potential for abuse than other Schedule I drugs.
The legal status of unauthorised actions with psilocybin mushrooms varies worldwide. While the United Nations Convention on Psychotropic Substances requires its members to prohibit psilocybin, the mushrooms containing the drug were not specifically included in the convention. Many countries have some level of regulation or prohibition of psilocybin mushrooms, and in some jurisdictions, psilocybin spores are legal to sell and possess because they do not contain the drugs psilocybin and psilocin.
In recent years, there has been a growing interest in the therapeutic potential of psilocybin mushrooms, particularly in the treatment of mental illnesses. Preliminary research studies suggest that psilocybin may be effective for smoking cessation and for disorders such as cancer-specific depression and anxiety. Researchers from Johns Hopkins University have been at the forefront of exploring innovative treatments using psilocybin and evaluating the safety and abuse potential of the drug.
These researchers suggest that if psilocybin clears phase III clinical trials, it should be reclassified as a Schedule IV drug, similar to prescription sleep aids, but with tighter control. Schedule IV drugs are those that have a lower potential for abuse and some accepted medical use. The researchers believe that reclassifying psilocybin as a Schedule IV drug would facilitate its path to clinical use and minimise logistical hurdles in the future. They recommend that when taken for a clinical reason, psilocybin should be administered in a healthcare setting monitored by a trained professional to minimise the potential for abuse or harm.
Mushroom Coffee: Does It Really Work?
You may want to see also
Frequently asked questions
Psilocybin and psilocin, the active substances in hallucinogenic mushrooms, are Schedule I drugs in the US. Schedule I drugs are defined as drugs with a high potential for abuse and no recognized medical uses. However, the mushrooms themselves are not specifically included in the 1971 United Nations Convention on Psychotropic Substances, and their legal status varies worldwide.
Schedule I drugs are defined by the United States Drug Enforcement Agency (DEA) as substances with a high potential for abuse and no currently accepted medical treatment use in the US. They are the only schedule of drugs that cannot be prescribed and are not readily available for clinical use.
Yes, researchers at Johns Hopkins suggest that if psilocybin clears phase III clinical trials, it should be reclassified as a Schedule IV drug, which has a lower potential for abuse and can be prescribed for medical use.











































