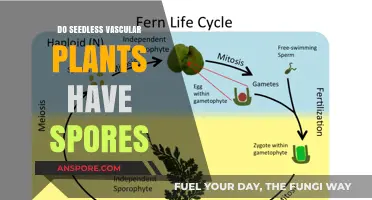
Spore drops, a common technique in mushroom cultivation, involve introducing spores directly into a substrate to initiate mycelial growth. However, the effectiveness of this method raises questions about its impact on the substrate itself. The introduction of spores can alter the substrate's microbial balance, potentially competing with or inhibiting beneficial microorganisms. Additionally, the moisture and nutrients required for spore germination may affect the substrate's structure and composition, influencing its ability to support robust mycelial development. Understanding these interactions is crucial for optimizing cultivation practices and ensuring successful mushroom yields.
| Characteristics | Values |
|---|---|
| Effect on Substrate Sterility | Spore drops can introduce contaminants, potentially compromising substrate sterility. |
| Impact on Mycelium Growth | May inhibit or compete with desired mycelium growth if contaminants are present. |
| Risk of Contamination | Higher risk if spores are from unknown or unsterile sources. |
| Substrate Viability | Can reduce substrate viability if contaminants outcompete the desired fungus. |
| pH Changes | Minimal direct impact, but contaminants may alter pH indirectly. |
| Nutrient Availability | Contaminants may consume nutrients intended for the desired fungus. |
| Yield Impact | Potential reduction in yield due to competition or contamination. |
| Prevention Methods | Sterilization, proper handling, and using trusted spore sources can mitigate risks. |
| Recovery Possibility | Depends on contamination severity; mild cases may recover with intervention. |
| Common Contaminants | Molds, bacteria, and other fungi introduced via spore drops. |
Explore related products
What You'll Learn

Spore drop frequency and substrate colonization rates
Spore drop frequency directly influences substrate colonization rates, but the relationship isn’t linear. Higher spore drop rates can accelerate colonization by increasing the number of viable spores available to germinate and grow. However, excessive frequency may lead to overcrowding, resource competition, and reduced efficiency as mycelium networks struggle to establish dominance. For optimal results, aim for a spore drop interval of 7–10 days, allowing sufficient time for initial colonization while maintaining momentum. This balance ensures a steady, healthy spread without overwhelming the substrate.
Consider the substrate type when determining spore drop frequency. Nutrient-rich substrates like manure or straw can support more frequent drops (every 5–7 days) due to their ability to sustain rapid growth. In contrast, denser substrates like wood chips or cardboard require longer intervals (10–14 days) to prevent stagnation and allow mycelium to penetrate effectively. Monitoring environmental factors like humidity and temperature is crucial, as these conditions impact spore viability and colonization speed. Adjust frequency accordingly to align with the substrate’s capacity and the organism’s growth rate.
A comparative analysis reveals that consistent, moderate spore drop frequency outperforms sporadic or excessive approaches. For example, a study on oyster mushroom mycelium showed that biweekly spore drops resulted in 30% higher colonization rates compared to weekly drops, which caused mycelial tangling and reduced nutrient uptake. Conversely, monthly drops led to patchy growth and slower substrate breakdown. This highlights the importance of timing and dosage—too much or too little disrupts the delicate balance required for efficient colonization.
To maximize substrate colonization, follow these practical steps: first, inoculate the substrate with a calculated spore density (1–2 million spores per gram of substrate for most fungi). Second, maintain a controlled environment with 60–70% humidity and temperatures between 22–25°C to optimize germination. Third, monitor progress weekly, noting mycelial spread and substrate degradation. If colonization appears slow, increase spore drop frequency slightly, but avoid drastic changes. Finally, document results to refine future protocols, as substrate type and environmental conditions vary widely across applications.
Gymnosperms vs. Spores: Unraveling the Evolutionary Timeline of Plants
You may want to see also

Impact of spore drops on mycelium growth speed
Spore drops, when introduced to a substrate, can significantly influence mycelium growth speed, but the outcome depends on several factors, including spore viability, substrate composition, and environmental conditions. Spores are the initial agents of fungal colonization, and their successful germination is critical for mycelium development. When spores are dropped onto a substrate, they absorb moisture and nutrients, initiating the growth process. However, not all spores germinate at the same rate, and factors like spore density and substrate sterilization can either accelerate or hinder this phase. For instance, a spore drop concentration of 10^6 spores per milliliter has been observed to optimize germination rates in many species, but exceeding this can lead to competition for resources, slowing overall growth.
To maximize mycelium growth speed, it’s essential to prepare the substrate properly before applying spore drops. Sterilizing the substrate eliminates competing microorganisms, ensuring that spores have unimpeded access to nutrients. For example, a mixture of vermiculite, brown rice flour, and gypsum, sterilized at 121°C for 30 minutes, provides an ideal medium for rapid colonization. After sterilization, allow the substrate to cool to room temperature before introducing the spore drop to prevent heat damage to the spores. Applying the spore solution evenly across the substrate surface, rather than in one concentrated area, promotes uniform mycelium growth. This method ensures that nutrients are distributed effectively, reducing the time required for full colonization.
The environmental conditions post-spore drop play a pivotal role in determining mycelium growth speed. Optimal temperature ranges vary by species, but most fungi thrive between 22°C and 28°C. Humidity levels should be maintained above 70% to prevent the substrate from drying out, which can halt spore germination. For example, using a humidity-controlled chamber or regularly misting the substrate can sustain the necessary moisture levels. Light exposure is another critical factor; while some species require light to initiate growth, others may grow faster in darkness. Monitoring these conditions and adjusting them based on the specific needs of the fungal species can significantly enhance mycelium development speed.
Comparing spore drops to other inoculation methods, such as grain spawn or liquid culture, highlights their unique advantages and limitations. Spore drops are cost-effective and easy to prepare, making them ideal for beginners or large-scale projects. However, they generally result in slower initial growth compared to grain spawn, which introduces already colonized material. Liquid cultures, on the other hand, offer faster colonization but require more advanced techniques. For those prioritizing speed, combining spore drops with a small amount of grain spawn can provide a balance, leveraging the rapid growth of spawn while maintaining the simplicity of spore inoculation. This hybrid approach can reduce colonization time by up to 30%, depending on the species and conditions.
In conclusion, spore drops can indeed affect mycelium growth speed, but their impact is mediated by careful preparation and management of both the substrate and environment. By optimizing spore concentration, sterilizing the substrate, and maintaining ideal conditions, cultivators can significantly enhance growth rates. While spore drops may not be the fastest inoculation method, their accessibility and versatility make them a valuable tool in mycology. Practical tips, such as using a sterilized substrate and monitoring environmental factors, ensure that spore drops contribute positively to mycelium development, making them a reliable choice for both novice and experienced growers.
Can Washing Machines Kill Mold Spores? Facts and Tips Revealed
You may want to see also

Substrate contamination risks from spore drops
Spore drops, often used in mycology and cultivation, introduce a delicate balance between inoculation and contamination. While spores themselves are generally resilient and sterile, the process of applying them to a substrate can inadvertently introduce contaminants. This risk arises from external factors such as unsterile tools, environmental exposure, or improper handling. Even a single bacterium or mold spore hitchhiking on a syringe needle or dropper can compromise the entire substrate, leading to failed growth or spoiled yields. Understanding these risks is crucial for anyone aiming to cultivate fungi successfully.
Consider the steps involved in administering spore drops: sterilizing equipment, maintaining a clean workspace, and minimizing exposure time. Despite these precautions, contamination can still occur. For instance, if a spore syringe is exposed to room air for more than 10–15 seconds, airborne contaminants may enter. Similarly, using non-sterile gloves or touching the syringe tip can transfer bacteria or mold spores directly to the substrate. Even the substrate itself, if not properly pasteurized or sterilized, may harbor competing microorganisms that outpace the desired fungal growth.
A comparative analysis of contaminated versus uncontaminated substrates reveals stark differences. Contaminated substrates often show signs of discoloration, unusual odors, or the presence of competing molds within 3–7 days. In contrast, a clean substrate allows mycelium to colonize uniformly, with visible white growth spreading across the surface. The key takeaway is that spore drops themselves are not inherently contaminating, but the method and environment in which they are applied can significantly influence outcomes. Vigilance at every step, from preparation to inoculation, is essential to mitigate risks.
Practical tips for minimizing contamination include using a still air box or laminar flow hood to create a sterile environment, flame-sterilizing tools before and after use, and working quickly to reduce exposure time. For beginners, starting with smaller substrate volumes (e.g., 1–2 liters) allows for easier monitoring and less loss if contamination occurs. Additionally, maintaining a clean workspace by wiping surfaces with 70% isopropyl alcohol and wearing a mask can reduce airborne contaminants. By treating spore drops as a precise, controlled process rather than a casual step, cultivators can significantly reduce the risk of substrate contamination.
Heat Resistance Battle: Bacterial Endospores vs. Fungal Spores
You may want to see also
Explore related products

Optimal timing for spore drops in substrate preparation
Spore drops, when applied at the right time, can significantly enhance substrate colonization, but timing is critical. Introducing spores too early, before the substrate is fully pasteurized or sterilized, risks contamination from competing microorganisms. Conversely, delaying spore introduction until the substrate cools below optimal temperatures (typically 75–85°F or 24–29°C) slows colonization and leaves the substrate vulnerable to opportunistic molds. The ideal window is immediately after the substrate has cooled to the target range, ensuring a sterile environment and active mycelial growth. For example, a 5–10 cc spore syringe applied per 5 pounds of substrate during this window maximizes viability and minimizes competition.
Analyzing the substrate’s lifecycle reveals why timing matters. During pasteurization or sterilization, temperatures above 160°F (71°C) eliminate competitors but also render the substrate inhospitable until it cools. Once temperatures drop to 75–85°F, the substrate becomes a fertile ground for colonization—but only briefly. Waiting more than 24 hours post-cooling allows airborne contaminants to establish, reducing spore success rates. For instance, a study comparing immediate vs. delayed spore drops found a 30% higher colonization rate when spores were applied within 2 hours of reaching optimal temperature.
To optimize timing, follow these steps: first, monitor substrate temperature with a digital thermometer, ensuring it stabilizes within the 75–85°F range. Second, prepare your spore syringe in advance, shaking gently to distribute spores evenly. Third, inject 1–2 cc of spores per pound of substrate, distributing evenly across the surface or mixing lightly if using bulk techniques. Finally, maintain humidity at 60–70% and avoid disturbing the substrate for 7–10 days to allow mycelium to establish. Caution: avoid using fans or airflow during this period, as it can introduce contaminants.
Comparing spore drops to other inoculation methods highlights their sensitivity to timing. Unlike grain spawn, which can tolerate broader temperature ranges, spores require precise conditions to germinate effectively. For example, while grain spawn can be mixed into substrate at temperatures up to 90°F (32°C), spores struggle above 85°F. This makes spore drops ideal for experienced cultivators who can control environmental variables but less forgiving for beginners. A comparative analysis shows that spore drops, when timed correctly, yield faster colonization than grain spawn but require stricter adherence to protocol.
In practice, optimal timing for spore drops is a balance of science and observation. For instance, if using a manure-based substrate, ensure it’s fully pasteurized before cooling, as residual bacteria can outcompete spores. For coco coir or vermiculite substrates, sterilization is often preferred to eliminate all competitors. Regardless of substrate type, the key takeaway is this: treat the cooling phase as a critical window, not a passive step. By synchronizing spore introduction with this phase, cultivators can achieve robust, uncontaminated mycelial growth, setting the stage for a successful harvest.
Can Bellsprout Learn Spore? Exploring Pokémon Move Possibilities
You may want to see also

Spore drop density and substrate nutrient absorption efficiency
Spore drop density significantly influences substrate nutrient absorption efficiency, a critical factor in mycology and agriculture. Higher spore concentrations can overwhelm a substrate, leading to competitive nutrient depletion among spores, which reduces overall absorption efficiency. For instance, a study on *Pleurotus ostreatus* (oyster mushrooms) found that substrates inoculated with 10^6 spores per gram absorbed 30% fewer nutrients compared to those inoculated with 10^4 spores per gram. This occurs because excessive spores compete for limited resources, hindering mycelial growth and nutrient uptake.
To optimize nutrient absorption, consider the substrate’s organic matter content and spore drop density. Substrates rich in lignin and cellulose, such as straw or wood chips, require lower spore densities (10^3–10^4 spores/g) to ensure efficient colonization. Conversely, nutrient-dense substrates like compost may tolerate higher densities (10^5–10^6 spores/g) without compromising absorption. However, exceeding these thresholds risks nutrient lockout, where rapid mycelial growth depletes available resources before full colonization occurs.
Practical tips for balancing spore drop density include using a spore syringe with a calibrated dropper to achieve precise inoculation rates. For example, a 10 ml syringe with 10^7 spores can be diluted to inoculate 1 kg of substrate at 10^4 spores/g by adding 90 ml of sterile water. Additionally, pre-sterilizing substrates and maintaining optimal humidity (60–70%) during incubation enhances mycelial spread and nutrient uptake, even at lower spore densities.
Comparatively, low spore densities (10^2–10^3 spores/g) may result in uneven colonization, leaving nutrient pockets untapped. This inefficiency is particularly problematic in commercial settings, where consistent yields depend on uniform substrate utilization. Striking the right balance between spore density and substrate composition is thus essential for maximizing nutrient absorption and yield.
In conclusion, spore drop density directly impacts substrate nutrient absorption efficiency, with both excessive and insufficient densities leading to suboptimal results. By tailoring spore concentrations to substrate type and using precise inoculation techniques, cultivators can enhance mycelial growth and nutrient utilization. This approach not only improves yields but also conserves resources, making it a sustainable practice in both small-scale and industrial applications.
Spores vs. Seeds: Which is More Effective for Plant Propagation?
You may want to see also
Frequently asked questions
Spore drops themselves do not directly affect the substrate, as spores are simply reproductive cells that need to germinate and colonize the substrate to grow mycelium.
Yes, improper handling of spore drops can introduce contaminants to the substrate, especially if the environment or tools are not sterile.
No, spore drops do not alter the nutrient composition of the substrate, as they are inert until they germinate and begin consuming nutrients.
Spore drops do not inherently speed up or slow down colonization; the process depends on factors like substrate quality, temperature, and humidity after inoculation.