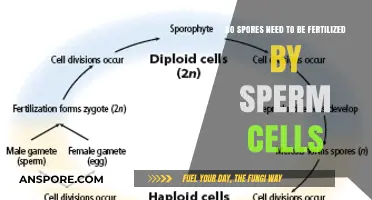
Spores and their vegetative counterparts in organisms like fungi and bacteria share the same DNA, as spores are typically produced through asexual reproduction mechanisms such as mitosis or budding. This process ensures that the genetic material is faithfully replicated, resulting in spores that are genetically identical to the parent organism in its vegetative state. However, in some cases, genetic variation can arise due to mutations or horizontal gene transfer, but under normal circumstances, spores retain the same DNA as their vegetative parent, allowing them to serve as a means of survival, dispersal, and reproduction without altering the organism's genetic identity.
| Characteristics | Values |
|---|---|
| DNA Content | Spores generally have the same DNA as their vegetative state, as they are produced through asexual reproduction (e.g., sporulation in bacteria, fungi, and plants). |
| Genetic Stability | Spores maintain genetic stability, preserving the DNA sequence of the parent organism during dormancy or dispersal. |
| Chromosome Number | Spores typically have the same chromosome number as the vegetative state, unless undergoing meiosis (e.g., in fungal spores). |
| Ploidy Level | In most cases, spores are haploid (e.g., fungal spores) if produced via meiosis, but bacterial endospores remain diploid, matching the vegetative state. |
| Mutation Rate | Spores may accumulate mutations over time due to environmental stress, but initially, their DNA is identical to the parent. |
| Epigenetic Changes | Spores may exhibit epigenetic modifications (e.g., DNA methylation, histone modifications) that differ from the vegetative state, affecting gene expression without altering DNA sequence. |
| Repair Mechanisms | Spores often have enhanced DNA repair mechanisms to ensure genetic integrity during dormancy or harsh conditions. |
| Genome Size | Genome size remains consistent between spores and the vegetative state, unless horizontal gene transfer or mutations occur post-sporulation. |
| Gene Expression | Spores may have distinct gene expression profiles compared to the vegetative state, optimized for survival and germination. |
| Exceptions | Some organisms (e.g., certain fungi) may undergo genetic recombination during spore formation, leading to slight DNA differences. |
Explore related products
What You'll Learn
- DNA Stability in Spores: Do spores maintain identical DNA structure during dormancy compared to their vegetative state
- Genetic Variation: Are there mutations or changes in spore DNA versus vegetative cells
- DNA Repair Mechanisms: How do spores preserve DNA integrity during harsh conditions
- Vegetative vs. Spore DNA Expression: Do gene expression patterns differ between spores and vegetative forms
- Heritability of Traits: Are traits from vegetative cells passed unchanged to spores

DNA Stability in Spores: Do spores maintain identical DNA structure during dormancy compared to their vegetative state?
Spores, the resilient survival structures of certain bacteria, fungi, and plants, are renowned for their ability to endure extreme conditions. But what happens to their DNA during this dormant state? Does it remain unchanged, or does dormancy introduce alterations? Understanding DNA stability in spores is crucial for fields like biotechnology, where spores are used for preservation and production, and for assessing their role in spreading antibiotic resistance or pathogens.
Spores achieve remarkable DNA stability through a combination of protective mechanisms. Firstly, their DNA is compacted into a highly condensed state, often associated with protective proteins like small, acid-soluble spore proteins (SASPs). This condensation shields DNA from damaging agents like UV radiation and desiccation. Secondly, spores exhibit elevated levels of DNA repair enzymes, enabling them to fix any damage that does occur during dormancy. Studies have shown that spore DNA can remain remarkably stable for centuries, with some bacterial spores retaining viable DNA even after being trapped in amber for millions of years.
While spores excel at DNA preservation, complete identity with their vegetative state DNA isn't guaranteed. Sporulation itself can induce subtle changes. For instance, DNA methylation patterns, which influence gene expression, can differ between vegetative cells and spores. Additionally, rare mutations can occur during spore formation or due to residual DNA damage not fully repaired before dormancy. These changes are typically minor and don't compromise spore viability, but they highlight that dormancy isn't a completely static state for DNA.
Research into DNA stability in spores has practical implications. In biotechnology, understanding these mechanisms can lead to improved methods for preserving genetically modified organisms or valuable microbial strains. Conversely, knowing the potential for DNA changes during sporulation is crucial for risk assessment in environmental and health contexts, particularly regarding the spread of antibiotic resistance genes harbored by spores.
Further research is needed to fully understand the dynamics of DNA stability in spores. Investigating the specific mechanisms of DNA repair during dormancy and the factors influencing mutation rates in spores will provide valuable insights. Additionally, studying DNA changes in spores exposed to different environmental stressors will enhance our ability to predict their behavior in various contexts. By unraveling the secrets of DNA stability in spores, we can harness their remarkable resilience while mitigating potential risks.
Flowering Plants and Spores: Unraveling the Myth of Their Reproduction
You may want to see also

Genetic Variation: Are there mutations or changes in spore DNA versus vegetative cells?
Spores, the resilient survival structures of certain organisms, are often assumed to be genetically identical to their vegetative counterparts. However, recent studies challenge this notion, revealing that genetic variation can occur during the transition from vegetative growth to spore formation. This raises the question: do spores harbor mutations or DNA changes that differentiate them from their parent cells?
The Sporulation Process: A Hotbed for Genetic Shuffling
Sporulation, the process of spore formation, involves a complex series of cellular events. In bacteria like *Bacillus subtilis*, DNA replication and segregation occur during sporulation, providing opportunities for mutations to arise. Research shows that spore DNA can differ from vegetative DNA due to:
- Replication errors: DNA polymerase mistakes during replication can introduce point mutations.
- Recombination events: Homologous recombination, a process more frequent during sporulation, can lead to genetic shuffling and novel allele combinations.
- Stress-induced mutations: Environmental stressors, such as nutrient deprivation or oxidative stress, can increase mutation rates during sporulation.
For instance, a study on *B. subtilis* found that spores exhibited a 10-fold higher mutation rate compared to vegetative cells, with an average of 1-2 mutations per genome per generation.
Implications for Spore Function and Evolution
The presence of genetic variation in spores has significant implications for their function and evolutionary potential. In fungi like *Neurospora crassa*, spore DNA diversity contributes to:
- Enhanced adaptability: Spores with unique genetic profiles may have a selective advantage in changing environments.
- Disease resistance: Genetic variation can provide spores with resistance to antibiotics or host immune responses.
- Speciation: Accumulated mutations in spores can lead to the emergence of new species over time.
A notable example is the discovery of spore variants in *Aspergillus fumigatus* with increased resistance to antifungal drugs, highlighting the clinical relevance of spore genetic variation.
Detecting and Quantifying Spore Genetic Variation
To study spore genetic variation, researchers employ techniques such as:
- Whole-genome sequencing: Comparing spore and vegetative DNA sequences to identify mutations and structural variations.
- PCR-based methods: Targeted amplification of specific genes or regions to detect mutations or recombination events.
- Fluctuation analysis: Measuring mutation rates in spores versus vegetative cells by assessing the frequency of antibiotic-resistant colonies.
For accurate results, it's essential to:
- Use synchronized cell cultures to ensure comparable growth stages.
- Replicate experiments with at least 3-5 biological replicates to account for variability.
- Apply appropriate bioinformatics tools to analyze sequencing data, such as variant callers (e.g., GATK) and assembly software (e.g., SPAdes).
Practical Considerations and Future Directions
Understanding spore genetic variation has practical applications in fields like biotechnology, medicine, and agriculture. For example:
- In fermentation industries, spore variants with improved productivity or stress tolerance can be selected for.
- In medicine, knowledge of spore genetic diversity can inform the development of more effective antifungal therapies.
Future research should focus on:
- Elucidating the molecular mechanisms underlying spore mutation rates.
- Investigating the role of epigenetic modifications in spore genetic variation.
- Developing predictive models to assess the impact of environmental factors on spore DNA changes.
By unraveling the complexities of spore genetic variation, we can harness the potential of these remarkable structures and mitigate their negative effects, such as drug resistance and food spoilage.
Mold Spores and Sore Throats: Uncovering the Hidden Connection
You may want to see also

DNA Repair Mechanisms: How do spores preserve DNA integrity during harsh conditions?
Spores, the resilient survival structures of certain bacteria, fungi, and plants, face extreme conditions that would destroy most life forms. Yet, they emerge unscathed, often with their DNA intact. How do they achieve this? The answer lies in a sophisticated arsenal of DNA repair mechanisms specifically tailored for dormancy and stress resistance.
Unlike their metabolically active vegetative counterparts, spores prioritize DNA preservation over replication and growth. Their DNA is condensed and protected within a protein matrix, minimizing damage from desiccation, radiation, and reactive oxygen species. This physical shielding is just the first line of defense.
One key mechanism is the enhanced activity of DNA repair enzymes within the spore. For instance, spores exhibit elevated levels of photolyase, an enzyme that directly reverses UV-induced DNA damage. This is particularly crucial for spores exposed to sunlight, where UV radiation poses a constant threat. Additionally, spores possess efficient base excision repair (BER) pathways, which target and correct damaged DNA bases caused by oxidation and alkylation. Studies have shown that BER activity in spores can be up to 10-fold higher than in vegetative cells, highlighting its importance in maintaining DNA integrity during dormancy.
Some spores even employ a unique repair strategy called "spore photoproduct lyase" (SPL) repair. This mechanism specifically targets DNA damage caused by UV light, further demonstrating the specialized nature of spore DNA repair systems.
Interestingly, spores don't rely solely on internal repair mechanisms. They also benefit from the protective environment they create. The thick spore coat acts as a physical barrier, shielding the DNA from external stressors. Additionally, the low metabolic activity within the spore reduces the production of reactive oxygen species, minimizing internal DNA damage.
Understanding these DNA repair mechanisms in spores has significant implications. It not only sheds light on the remarkable resilience of life but also inspires the development of novel strategies for preserving biological materials and developing radiation-resistant technologies. By deciphering the secrets of spore DNA protection, we unlock new possibilities for safeguarding genetic information in extreme environments, both on Earth and beyond.
Understanding Spores: Mitosis or Meiosis Production Explained
You may want to see also
Explore related products
$35.12 $36.99

Vegetative vs. Spore DNA Expression: Do gene expression patterns differ between spores and vegetative forms?
Spores and their vegetative counterparts share the same DNA sequence, yet their gene expression patterns diverge dramatically, reflecting their distinct biological roles. In the vegetative state, organisms prioritize growth, metabolism, and reproduction, activating genes associated with these functions. Spores, however, enter a dormant, stress-resistant phase, requiring a unique gene expression profile to survive harsh conditions. This differential expression is regulated by epigenetic modifications, transcription factors, and environmental cues, ensuring that each form thrives in its specific ecological niche.
Consider the example of *Bacillus subtilis*, a well-studied bacterium that forms endospores. During sporulation, genes encoding for spore coat proteins, such as *cotE* and *cotB*, are upregulated, while those involved in vegetative growth, like *dnaA* and *ftsZ*, are downregulated. This shift in gene expression is orchestrated by sigma factors, such as σ^H^ and σ^G^, which bind to specific promoters and activate sporulation-specific genes. Conversely, in the vegetative state, sigma factor σ^A^ dominates, driving the expression of genes essential for binary fission and nutrient uptake.
To investigate these differences experimentally, researchers often employ RNA-seq or microarray analyses to compare transcriptomes between spores and vegetative cells. For instance, a study on *Aspergillus nidulans* revealed that over 20% of its genome is differentially expressed between the two states, with spore-specific genes involved in stress resistance and dormancy maintenance. Practical tips for such experiments include synchronizing sporulation cultures to ensure uniformity and using stringent statistical thresholds to identify significant gene expression changes.
From an evolutionary perspective, the divergence in gene expression between spores and vegetative forms is a survival strategy. Spores act as a genetic reservoir, preserving the organism’s DNA during unfavorable conditions, while vegetative cells exploit resources during optimal environments. This duality ensures species longevity and adaptability. For instance, fungal spores can remain viable for decades, awaiting the right conditions to germinate, while their vegetative hyphae rapidly colonize nutrient-rich substrates.
In applied fields, understanding these differences has practical implications. For example, in agriculture, manipulating spore gene expression could enhance crop resilience to drought or disease. In medicine, targeting spore-specific genes in pathogens like *Clostridioides difficile* could lead to novel antimicrobial therapies. By deciphering the unique gene expression patterns of spores and vegetative forms, scientists can harness their distinct properties for innovation and problem-solving.
Beyond Fungi: Exploring Other Organisms Capable of Producing Spores
You may want to see also

Heritability of Traits: Are traits from vegetative cells passed unchanged to spores?
Spores, the resilient survival structures of certain organisms, are often assumed to be genetic clones of their vegetative parent cells. However, this assumption oversimplifies the complex relationship between vegetative cells and spores. While spores typically inherit the same DNA sequence as their vegetative counterparts, the expression of traits can differ significantly due to epigenetic modifications, environmental influences, and, in some cases, genetic recombination during spore formation.
Consider the life cycle of fungi, where vegetative cells (hyphae) produce spores through processes like meiosis or mitosis. In meiosis, genetic recombination occurs, introducing variability into the spore’s DNA. For example, in *Aspergillus nidulans*, meiotic recombination during sexual spore (ascospore) formation ensures genetic diversity, even though the spores inherit DNA from the same parent. Conversely, asexual spores (conidia) are genetically identical to the vegetative cells but may exhibit phenotypic differences due to epigenetic changes, such as DNA methylation or histone modification, which alter gene expression without changing the DNA sequence.
Epigenetic mechanisms play a critical role in trait heritability from vegetative cells to spores. In plants like ferns, sporophytes (vegetative phase) produce spores via meiosis, but the traits expressed in the gametophyte (spore phase) are influenced by epigenetic marks established in the sporophyte. For instance, studies in *Arabidopsis thaliana* show that small RNA molecules can be passed from vegetative cells to spores, affecting gene silencing in the next generation. This means that while the DNA sequence remains unchanged, the expression of traits can vary, leading to phenotypic differences between the vegetative state and the spore-derived organism.
Practical implications of this phenomenon are evident in agriculture and biotechnology. For example, in crop plants like wheat or rice, understanding how traits are inherited from vegetative tissues to spores (pollen or ovules) is crucial for breeding programs. Epigenetic modifications can affect traits like drought resistance or yield, even if the DNA sequence is identical. Farmers and breeders must consider not only the genetic makeup but also the epigenetic state of spores to predict and manipulate trait expression in subsequent generations.
In conclusion, while spores generally inherit the same DNA as their vegetative parent cells, traits are not always passed unchanged. Genetic recombination during spore formation and epigenetic modifications can introduce variability in trait expression. This nuanced understanding is essential for fields like microbiology, botany, and agriculture, where the heritability of traits from vegetative cells to spores directly impacts outcomes such as disease resistance, crop yield, and evolutionary adaptability.
Can Flowers Bloom from Spores in Subzero Temperatures?
You may want to see also
Frequently asked questions
Yes, spores typically contain the same DNA as the organism in its vegetative state, as they are produced through asexual reproduction or specialized cell division processes.
While rare, mutations can occur during spore formation or dormancy, potentially leading to slight DNA differences from the parent organism in its vegetative state.
In most cases, spores are genetically identical to the parent organism, but exceptions exist, such as in certain fungi where genetic recombination can occur during spore production.
Generally, the DNA in spores remains stable during dormancy, but changes can occur during germination due to environmental factors or repair mechanisms, though the core genetic material remains the same.