Bacillus, a genus of Gram-positive, rod-shaped bacteria, is widely recognized for its ability to form highly resistant endospores, which allow these organisms to survive in harsh environmental conditions. However, while spore formation is a hallmark characteristic of many Bacillus species, it is not universal across the entire genus. The question of whether all Bacillus species are spore formers is an important one, as it highlights the diversity within this group and the need for precise taxonomic and physiological distinctions. Understanding which Bacillus species produce spores and which do not is crucial for applications in biotechnology, medicine, and environmental science, where the spore-forming capability often dictates their utility and behavior.
| Characteristics | Values |
|---|---|
| Spore Formation | Not all Bacillus species form spores. While many are endospore-forming, some strains do not produce spores under any conditions. |
| Genus Bacillus | A large, diverse genus of Gram-positive, rod-shaped bacteria. Most are aerobic or facultatively anaerobic. |
| Endospore Location | In spore-forming Bacillus, endospores are located within the vegetative cell and are highly resistant to heat, radiation, and chemicals. |
| Examples of Spore Formers | Bacillus anthracis, Bacillus cereus, Bacillus subtilis. |
| Examples of Non-Spore Formers | Some strains of Bacillus licheniformis and Bacillus pumilus may not form spores under certain conditions. |
| Environmental Survival | Spore-forming Bacillus can survive in harsh environments for extended periods, while non-spore formers are less resilient. |
| Medical and Industrial Relevance | Spore-forming Bacillus species are significant in medicine (e.g., anthrax) and industry (e.g., enzyme production), while non-spore formers have limited applications. |
| Genetic Basis | Spore formation is controlled by specific genes (e.g., spo genes), which may be absent or inactive in non-spore-forming strains. |
| Laboratory Identification | Spore formation is confirmed via sporulation assays, phase-contrast microscopy, or staining techniques like the Schaeffer-Fulton stain. |
Explore related products
What You'll Learn
- Spore Formation Mechanism: How Bacillus species initiate and complete the sporulation process under stress
- Non-Spore Forming Strains: Identification of Bacillus strains that lack spore-forming capabilities despite genetic potential
- Environmental Triggers: Factors like nutrient depletion and pH changes inducing spore formation in Bacillus
- Spore Structure: Key components of Bacillus spores, including cortex, coat, and core
- Clinical Significance: Role of spore formation in Bacillus species' survival and pathogenicity in healthcare settings

Spore Formation Mechanism: How Bacillus species initiate and complete the sporulation process under stress
Not all Bacillus species are spore formers, but those that do exhibit this remarkable ability have evolved a sophisticated mechanism to survive harsh environmental conditions. When faced with nutrient deprivation, extreme temperatures, or other stressors, certain Bacillus species, such as *Bacillus subtilis* and *Bacillus anthracis*, initiate a complex process called sporulation. This process transforms the vegetative cell into a highly resistant spore, capable of enduring conditions that would otherwise be lethal. Understanding the sporulation mechanism is crucial for fields like microbiology, biotechnology, and medicine, as it sheds light on bacterial survival strategies and potential applications in spore-based technologies.
The sporulation process in Bacillus species is tightly regulated and involves a series of well-coordinated steps. It begins with the activation of a phosphorelay system, a signaling cascade triggered by stress signals such as nutrient limitation. This system ultimately leads to the activation of the master regulator Spo0A, which orchestrates the expression of genes required for sporulation. As the process progresses, the cell undergoes asymmetric division, forming a smaller forespore and a larger mother cell. The mother cell then engulfs the forespore, creating a double-membrane structure. This engulfment is a critical step, as it isolates the developing spore from the external environment, allowing for the synthesis of protective layers like the cortex and coat.
One of the most fascinating aspects of spore formation is the assembly of the spore’s protective layers. The cortex, composed of modified peptidoglycan, provides mechanical strength and resistance to heat, while the coat acts as a barrier against enzymes and chemicals. In some species, an additional layer called the exosporium is formed, offering further protection and surface properties. For example, *Bacillus anthracis* spores are encased in an exosporium that aids in environmental persistence and host interaction. The precise timing and coordination of these layers’ formation are essential for the spore’s viability and resilience.
Practical applications of understanding sporulation mechanisms are vast. In biotechnology, spores are used as delivery vehicles for vaccines, enzymes, and probiotics due to their stability. For instance, *Bacillus subtilis* spores are employed in the production of bioinsecticides, where their ability to withstand environmental stresses ensures long-term efficacy. In medicine, studying sporulation helps in developing strategies to combat spore-forming pathogens like *Clostridioides difficile* and *Bacillus anthracis*. Researchers are also exploring ways to disrupt sporulation pathways to prevent spore formation in harmful bacteria, potentially leading to new antimicrobial therapies.
To harness the potential of Bacillus spores, it’s essential to consider specific conditions that optimize sporulation. For laboratory cultures, nutrient-limited media, such as Difco Sporulation Medium, can induce sporulation in *Bacillus subtilis* within 24–48 hours. Maintaining a pH range of 7.0–7.5 and a temperature of 37°C typically yields the highest spore counts. For industrial applications, scaling up sporulation requires careful control of oxygen levels and agitation to ensure uniform spore formation. Understanding these parameters allows for the efficient production of spores tailored to specific needs, whether for research, agriculture, or healthcare.
Effective Milky Spore Application: A Step-by-Step Guide for Lawn Grub Control
You may want to see also

Non-Spore Forming Strains: Identification of Bacillus strains that lack spore-forming capabilities despite genetic potential
Not all Bacillus strains form spores, despite possessing the genetic machinery to do so. This phenomenon raises intriguing questions about the regulatory mechanisms governing sporulation and the environmental cues that trigger it. While Bacillus species are renowned for their ability to produce highly resistant endospores, certain strains defy this expectation, remaining vegetative under conditions that typically induce sporulation. Identifying these non-spore-forming strains is crucial for understanding the variability within the genus and for practical applications in biotechnology and microbiology.
To identify non-spore-forming Bacillus strains, researchers employ a combination of genetic analysis and phenotypic assays. PCR-based methods targeting sporulation-specific genes, such as *spo0A* or *sigE*, confirm the presence of the genetic potential for sporulation. However, the absence of spore formation under standard sporulation-inducing conditions (e.g., nutrient depletion or high salinity) indicates a disconnect between genetic capability and phenotypic expression. For instance, some strains may harbor mutations in key regulatory genes or exhibit altered expression patterns that suppress sporulation pathways. Culturing these strains on sporulation medium (e.g., DSMZ medium 92) and examining them microscopically for spore morphology provides a definitive phenotypic assessment.
One practical example involves Bacillus subtilis, a well-studied spore-former. Certain laboratory strains, such as B. subtilis 168, exhibit reduced sporulation efficiency due to genetic modifications or prolonged cultivation under non-sporulating conditions. These strains retain sporulation genes but fail to produce spores unless specific triggers, like overexpression of *spo0A*, are introduced. Similarly, environmental isolates of Bacillus may lack spore formation due to adaptations to stable, nutrient-rich habitats where sporulation is energetically unfavorable. For instance, strains isolated from soil with consistent organic matter may prioritize vegetative growth over spore production.
The identification of non-spore-forming Bacillus strains has significant implications for industrial applications. In biotechnology, spore-forming ability can complicate processes like fermentation or enzyme production by introducing variability in cell viability and product yield. Non-spore-forming strains offer a more predictable and controllable system for biomanufacturing. For example, in the production of amylases or proteases, using non-spore-forming Bacillus strains ensures consistent enzyme activity without the risk of sporulation-related downtime. However, caution must be exercised when selecting strains, as some may lack sporulation due to genetic instability or mutations that affect other desirable traits.
In conclusion, the identification of non-spore-forming Bacillus strains requires a nuanced approach combining genetic and phenotypic analyses. Understanding why certain strains fail to sporulate despite genetic potential sheds light on the complex regulatory networks governing this process. For researchers and industry professionals, these strains offer unique advantages in applications where spore formation is undesirable. By carefully characterizing and utilizing these strains, we can harness the versatility of Bacillus species while mitigating the challenges associated with sporulation.
Stun Spore vs. Electric Types: Does It Work in Pokémon Battles?
You may want to see also

Environmental Triggers: Factors like nutrient depletion and pH changes inducing spore formation in Bacillus
Bacillus species are renowned for their ability to form highly resistant spores, a survival mechanism triggered by specific environmental cues. Among these, nutrient depletion and pH changes stand out as critical factors that induce spore formation. When nutrients become scarce, Bacillus cells sense the shift and initiate a complex developmental program to ensure long-term survival. This process, known as sporulation, is a finely tuned response to environmental stress, allowing these bacteria to persist in harsh conditions where active growth is unsustainable.
Consider nutrient depletion as a primary trigger. In laboratory settings, researchers often induce sporulation by transferring Bacillus cultures from nutrient-rich media to minimal or starvation media. For instance, reducing the concentration of carbon sources like glucose from 1% to 0.05% can significantly accelerate spore formation in *Bacillus subtilis*. This mimics natural environments where resources are limited, such as soil or water bodies with fluctuating nutrient availability. The bacterium’s response is not merely passive; it actively detects nutrient scarcity through signaling pathways, such as the phosphorylation cascade involving the kinase A (KinA) protein, which activates the sporulation transcription factor Spo0A. This molecular switch underscores the bacterium’s ability to adapt proactively to environmental challenges.
PH changes represent another potent environmental trigger for spore formation in Bacillus. These bacteria thrive in neutral to slightly alkaline conditions but can initiate sporulation when exposed to extreme pH levels. For example, a drop in pH from 7.0 to 5.0 can induce sporulation in *Bacillus cereus*, a species commonly found in soil and food products. This response is particularly relevant in acidic environments, such as decaying organic matter or contaminated food, where pH fluctuations signal deteriorating conditions. The mechanism involves pH-sensitive proteins and two-component systems that relay environmental signals to the cell’s regulatory machinery, ultimately activating the sporulation pathway.
Practical applications of these environmental triggers are evident in industries like food preservation and biotechnology. For instance, controlling pH and nutrient levels in food processing can prevent spore formation in pathogenic Bacillus species, reducing the risk of foodborne illnesses. Conversely, in biotechnology, inducing sporulation under controlled conditions allows for the production of robust spores used in probiotics, biocontrol agents, or enzyme carriers. Understanding these triggers enables precise manipulation of Bacillus behavior, whether to inhibit unwanted sporulation or harness it for beneficial purposes.
In summary, nutrient depletion and pH changes are not mere stressors but specific signals that Bacillus species interpret to initiate spore formation. These environmental triggers highlight the bacterium’s remarkable adaptability and provide actionable insights for both controlling and utilizing sporulation in various contexts. By studying these mechanisms, scientists can develop strategies to manage Bacillus populations in natural and industrial settings, ensuring safety and efficiency in applications ranging from food safety to biotechnology.
Seeds vs. Spores: Unraveling the Unique Differences in Plant Reproduction
You may want to see also
Explore related products
$12.99 $15.99

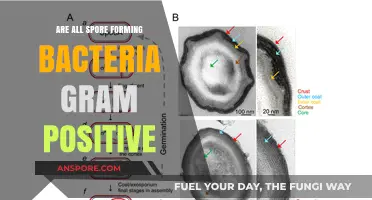
Spore Structure: Key components of Bacillus spores, including cortex, coat, and core
Bacillus spores are renowned for their resilience, capable of withstanding extreme conditions such as heat, radiation, and desiccation. This durability is largely attributed to their intricate spore structure, which consists of three key components: the cortex, coat, and core. Each layer plays a distinct role in protecting the dormant bacterial cell, ensuring its survival until favorable conditions return. Understanding these components not only sheds light on the spore's robustness but also highlights potential targets for antimicrobial strategies.
The cortex, positioned between the spore coat and the core, is a modified peptidoglycan layer that provides structural integrity and acts as a barrier against environmental stressors. Unlike the vegetative cell wall, the cortex is less cross-linked, allowing it to swell rapidly upon germination. This swelling facilitates the rehydration of the core, a critical step in reviving the dormant bacterium. Interestingly, the cortex is also responsible for the spore's resistance to heat, as it prevents the core from dehydrating under high temperatures. For instance, in industrial sterilization processes, spores with a well-developed cortex require prolonged exposure to steam (typically 121°C for 15–30 minutes) to ensure complete inactivation.
Encasing the cortex is the spore coat, a proteinaceous layer composed of over 70 different proteins arranged in a complex, multilayered structure. The coat serves as the primary defense against physical and chemical insults, including enzymes, detergents, and UV radiation. Its hydrophobic nature also prevents water uptake, contributing to the spore's desiccation tolerance. Notably, the coat’s composition varies among Bacillus species, which explains differences in spore resistance. For example, Bacillus anthracis spores possess a unique exosporium layer atop the coat, enhancing their environmental persistence. This variability underscores the coat’s role as a species-specific adaptation to diverse habitats.
At the heart of the spore lies the core, a dehydrated, metabolically dormant cell containing the bacterial genome, ribosomes, and essential enzymes. The core’s low water content (approximately 20–25% compared to 80% in vegetative cells) is critical for its stability, as it minimizes chemical reactions that could damage cellular components. Additionally, the core is protected by dipicolinic acid (DPA), a calcium-chelating molecule that binds free water and stabilizes DNA and proteins. During germination, DPA is released, and the core rehydrates, signaling the resumption of metabolic activity. This process is so efficient that spores can revive within minutes under optimal conditions, such as nutrient availability and appropriate temperature (e.g., 37°C for many Bacillus species).
In summary, the spore structure of Bacillus is a marvel of evolutionary engineering, with the cortex, coat, and core each contributing uniquely to survival. The cortex provides structural support and heat resistance, the coat offers protection against environmental hazards, and the core safeguards genetic material in a dormant state. By dissecting these components, researchers can develop targeted interventions, such as coat protein inhibitors or DPA-depleting agents, to combat spore-forming pathogens. For practical applications, understanding spore structure is essential for industries like food safety, pharmaceuticals, and biotechnology, where spore inactivation is a critical challenge.
Can Alcohol Effectively Eliminate Mold Spores? Facts and Myths Revealed
You may want to see also

Clinical Significance: Role of spore formation in Bacillus species' survival and pathogenicity in healthcare settings
Spore formation is a defining characteristic of many Bacillus species, enabling their survival in harsh environments. However, not all Bacillus species are spore formers, and this distinction is critical in understanding their clinical significance in healthcare settings. For instance, Bacillus anthracis, the causative agent of anthrax, is a well-known spore former, while Bacillus subtilis, often used as a probiotic, also exhibits this capability. In contrast, some Bacillus species, such as Bacillus cereus, can form spores but are primarily associated with foodborne illnesses rather than healthcare-associated infections (HAIs). This variability underscores the importance of identifying spore-forming Bacillus species in clinical contexts, as their ability to persist in the environment significantly impacts infection control strategies.
The role of spore formation in pathogenicity cannot be overstated, particularly in healthcare settings where immunosuppressed patients are at heightened risk. Spores are highly resistant to physical and chemical stressors, including heat, radiation, and disinfectants commonly used in hospitals. For example, spores of Bacillus anthracis can survive in soil for decades, and once inhaled, they germinate into vegetative cells, leading to systemic infection. Similarly, spores of Bacillus cereus have been implicated in outbreaks of HAIs, such as bacteremia and meningitis, especially in neonatal intensive care units. The ability of these spores to adhere to medical devices, such as catheters and ventilators, further complicates infection prevention efforts. Effective disinfection protocols must account for spore resistance, often requiring sporicidal agents like hydrogen peroxide vapor or peracetic acid, which are more potent than standard disinfectants.
From a practical standpoint, healthcare professionals must adopt a multi-faceted approach to mitigate the risks associated with spore-forming Bacillus species. First, environmental surveillance is essential to identify reservoirs of contamination, particularly in high-risk areas like operating rooms and ICUs. Second, stringent hand hygiene practices, using alcohol-based hand rubs with sporicidal activity, are critical in preventing spore transmission. Third, proper sterilization of medical equipment, such as autoclaving at 121°C for 15–30 minutes, ensures the destruction of spores. For patients at high risk, such as those undergoing chemotherapy or organ transplantation, proactive measures like isolation precautions and environmental decontamination should be implemented. Additionally, educating staff about the unique challenges posed by spore-forming pathogens is vital for fostering a culture of vigilance.
Comparatively, the clinical management of infections caused by spore-forming Bacillus species differs from that of non-spore formers. While antibiotics like vancomycin and carbapenems are effective against vegetative cells, spores require specific treatment strategies. For instance, anthrax infections necessitate early administration of antibiotics such as ciprofloxacin or doxycycline, combined with antitoxin therapy to neutralize the effects of bacterial exotoxins. In contrast, Bacillus cereus infections often resolve with supportive care, though severe cases may require targeted antibiotic therapy. The emergence of antibiotic resistance in some Bacillus species, particularly to beta-lactams, further complicates treatment, emphasizing the need for judicious antibiotic use and ongoing antimicrobial stewardship programs in healthcare facilities.
In conclusion, the ability of certain Bacillus species to form spores plays a pivotal role in their survival and pathogenicity in healthcare settings. Understanding this mechanism is essential for developing effective infection control and treatment strategies. By implementing evidence-based practices, healthcare providers can minimize the risk of spore-related infections and protect vulnerable patient populations. As research continues to uncover the complexities of Bacillus spore biology, staying informed and adaptable will remain key to addressing this persistent clinical challenge.
Optimal Timing for Planting Morel Spores: A Comprehensive Guide
You may want to see also
Frequently asked questions
Yes, all Bacillus species are known to form endospores, which are highly resistant structures that allow them to survive harsh environmental conditions.
Bacillus bacteria can survive in vegetative form under favorable conditions, but spore formation is their key survival mechanism in adverse environments.
No, spore formation is a defining characteristic of the Bacillus genus, and all members are capable of producing endospores.
Bacillus spores are significant because they enable the bacteria to withstand extreme conditions such as heat, radiation, and desiccation, ensuring long-term survival.